Most Studies Indicate Popular GM Food Not as Safe as Conventional Counterpart
Background:
Genetically modified (GM) food safety is a controversial subject. Some use the narrative reviews Nicolia 2014 and Sanchez 2017 to claim that the consensus in the scientific literature is that all GM foods currently on the market are as safe as their conventional counterparts.
Purpose:
To examine feeding studies using animals comparable to humans fed GM soy with single event GTS 40-3-2, and health parameters, which were reviewed by Nicolia 2014 and Sanchez 2017. Since the claim is that all GM foods currently on the market are as safe as their conventional counterparts, if the consensus in the scientific literature is that even a single GM food on the market is less safe, then this claim must be rejected. We chose GTS 40-3-2 as it is one of the most grown, has the most animal feeding studies according to Sanchez 2017 and is one of the most approved GM foods internationally.
Data Sources and Selection:
Review of all sources purported to be used by Nicolia 2014 and Sanchez 2017 pertaining to relevant animal feeding studies using GM food GTS 40-3-2.
Data Extraction:
Relevant studies were identified which used GTS 40-3-2.
Results:
Sanchez 2017
71.4% of relevant studies reviewed in Sanchez 2017 suggest adverse effects or biomarkers indicative of adverse effects from the GTS 40-3-2 diet.
100% of the medium and long-term studies, of 6 months feeding duration or longer, reviewed in Sanchez 2017 suggest adverse effects or biomarkers indicative of adverse effects from the GTS 40-3-2 diet.
86.2% of the relevant studies that should have been reviewed in Sanchez 2017 suggest adverse effects or biomarkers indicative of adverse effects from the GTS 40-3-2 diet.
Nicolia 2014
90% of relevant studies reviewed by Nicolia et al. suggest adverse effects or biomarkers indicative of adverse effects from the GTS 40-3-2 diet.
100% of the medium and long-term studies, of 6 months feeding duration or longer, reviewed in Nicolia 2014 suggest adverse effects or biomarkers indicative of adverse effects from the GTS 40-3-2 diet.
94.7% of the relevant studies that should have been reviewed in Nicolia 2014 suggest adverse effects or biomarkers indicative of adverse effects from the GTS 40-3-2 diet.
Conclusion:
Based on the results of our systematic review, the claim that all GM foods currently on the market are as safe as their conventional counterparts is not supported by the weight of the scientific evidence. Instead, a clear consensus in the scientific literature regarding the popular GM food GTS 40-3-2, considered to be the most tested GM food, has emerged. Our systematic review of the scientific evidence indicates that in most of the relevant animal feeding studies there were adverse effects or biomarkers indicative of adverse effects reported.
These results are consistent with systematic reviews indicating an overwhelming consensus among health groups and individual health professionals on GM foods. The consensus among these health experts is that GM foods currently on the market cannot presently be considered as safe as their conventional counterparts (GMO Free Florida 2022, GMO Free Florida 2022a). Therefore, we call upon the health community, who are the experts on health, to continue to inform the public of the potential harms from GM foods and to choose non-GMO and organic foods to avoid those potential harms. We urge the governments of the world to impose a moratorium on all GM foods until each GM food has been demonstrated as safe in independent long-term and multigenerational chronic toxicity/carcinogenicity studies using both rodents and non-rodents comparable to humans. We also call upon all who have published papers claiming that there is a consensus that all GM foods on the market are safe to provide corrections, or formally retract their papers if necessary. Our systematic reviews indicate this claim is not supported by the consensus, nor does it appear this claim was ever supported by the consensus.
A precautionary approach should be taken especially since there is now a consensus among health groups and individual health professionals that GM foods currently on the market cannot be considered as safe as their conventional counterparts at this present time and a consensus in the scientific literature that some GM foods currently on the market may be unsafe compared to their conventional counterparts.
Disclaimer: Neither GMO Free Florida, our fiscal sponsor GMO Free USA, nor any person acting on behalf of GMO Free Florida or GMO Free USA are responsible for the use, actions or decisions taken as a result of the information in this report. The views expressed in this report are those of the unpaid volunteers who authored this report and are not necessarily the views of GMO Free Florida or GMO Free USA. The authors declare no conflicts of interest.
Introduction:
Genetically modified (GM) foods are also referred to as genetically engineered (GE) or bioengineered (BE) foods. These include foods modified through techniques such as transgenesis, intragenesis, cisgenesis, zinc finger proteins, transcription activator-like effector nucleases, and clustered, regularly interspaced, short, palindromic repeats. The process of genetic modification can result in unexpected consequences, potentially causing the plant to produce toxins, create foreign proteins, or other unanticipated results (Ho 2013, Wilson 2006, Dona 2009, Rang 2005, Mesnage 2016, Eckerstorfer 2019). GM food safety is, therefore, a controversial subject.
Animal feeding studies are one of the main lines of evidence used to claim GM foods currently on the market are as safe as conventional foods. For example, the American Association for the Advancement of Science Board of Director’s statement mentions animal feeding studies as evidence for the safety of GM foods (American Association for the Advancement of Science 2012). Narrative reviews which include animal feeding studies are often cited to claim there is a consensus that all GM foods currently on the market are as safe as their conventional counterparts. The narrative reviews Nicolia 2014 and Sanchez 2017 are some of the most cited reviews when making this claim (Blair 2020).
Glyphosate tolerant soy varieties have consistently been the most grown GM crop internationally (ISAAA 2019) with GTS 40-3-2 being the dominant event over most of that time (Bøhn 2014). GTS 40-3-2 continues to be one of the most grown GM soy events in countries such as the United States and Brazil where most of the GM soy is grown (Soga 2020). GTS 40-3-2 is also the most analyzed GM food, having the greatest number of animal health studies (Sanchez 2017), and is approved for consumption by the second most number of countries (ISAAA 2019). GM soy has been detected in foods sold in many countries around the world (Grazina 2017, Carvajal 2017, Sakr 2014, Elsanhoty 2013, Rosculete 2018, Nikolić 2009, Ujhelyi 2008, Viljoen 2006). Even in many countries that require GMO labeling, GM soy has been detected in unlabeled foods above the threshold for labeling (Grazina 2017, Rosculete 2018, Ujhelyi 2008, Nikolić 2009).
Since people in most countries are generally consuming GM soy, in many cases without even knowing, it would not be valid to just compare disease rates of different countries to look for harm specific to GM soy. For example, an examination of disease rates for North America compared with western Europe would not be valid, as both groups would generally be consuming GM soy. In the absence of human studies, and with significant challenges for epidemiological studies, animal feeding studies using animals comparable to humans likely provide the best available evidence of safety or harm from GM foods currently available. Therefore, we reviewed peer reviewed studies using animals comparable to humans, health parameters and single event 40-3-2 glyphosate tolerant soy feed which were published between 1993 and 2014. This was used to establish whether the consensus claim on GM food safety is supported by a systematic review of the scientific literature.
Systematic Review vs. Narrative Review
A systematic review includes a focused question, consistent standard for inclusion and exclusion criteria, a comprehensive search that can be replicated and a conclusion based on the weight of the scientific evidence. A narrative review can include or exclude studies in an arbitrary, or biased, and inconsistent manner to convey a specific narrative which reflects the author’s opinion. A narrative review does not need to be able to be replicated and the conclusion does not have to be based on the weight of the scientific evidence (Rys 2009). Since narrative reviews have a large potential to be biased, apply double standards and omit relevant studies, such reviews are not considered high quality evidence (UTHealth 2020). Therefore, narrative reviews should not be used as evidence to claim a consensus that all GM foods on the market are as safe as their conventional counterparts (Icahn Undated). Unlike Nicolia 2014 and Sanchez 2017, our systematic review can be replicated, does not apply inconsistent standards or omit relevant studies, or use other forms of bias. Our systematic review also relies on the weight of the scientific evidence to answer a focused question about the safety of one of the most popular GM foods on the market, GM soy GTS 40-3-2.
Sanchez Reviews
The results of our systematic reviews on the opinions of health groups and surveys of health practitioners (GMO Free Florida 2022, GMO Free Florida 2022a) appear to conflict with a recent narrative review (Sanchez 2017). The Sanchez 2017 review concluded that only 5% of the studies using GM foods reported adverse effects. Sanchez 2017 also concluded that a greater number of studies reporting adverse effects had a conflict of interest (COI) compared to those that did not report adverse effects. We attempted to determine the reason for these conflicting views by examining the Sanchez 2017 narrative review in detail. We focused specifically on relevant animal health studies using GM soy GTS 40-3-2 as Sanchez 2017 states this is the most analyzed GM food.
Results Cannot Be Verified or Replicated:
Sanchez 2017 reports using references cited on the internet by GMO Free USA, Coalition for a GM Free India and GM Watch, but does not identify the exact sources used. Therefore, it is not possible to verify or replicate many of the claims made. References cited in the internet could include numerous posts on Facebook, Twitter, and other social media platforms as well as websites. Since Sanchez 2017 does not provide any details as to what sources were actually reviewed, their claims cannot be verified and, therefore, their conclusions are unreliable.
Glyphosate Tolerant Soy GTS 40-3-2 Systematic Review:
We, therefore, took a fresh look at the data purported to be used in Sanchez 2017. In Sanchez 2015 it was declared that the category of “Animal health” had the most studies. We reviewed animal health studies limiting our search to in vivo studies using rodents, pigs, dogs, non-human primates and humans as subjects, however, no studies were found using dogs, non-human primates and humans. Such studies are one of the main lines of evidence used to claim GM foods currently on the market are as safe as conventional foods. Animal health studies also appear to represent the majority of the studies, and GM soy event 40-3-2 has been the most analyzed according to Sanchez 2017.
We, therefore, reviewed animal health studies that were published in peer reviewed journals during the timeframe used by Sanchez 2015 and found in the Sanchez 2015 and Sanchez 2017 reviews. We also used studies cited in the Internet by the Coalition for a GM Free India document “Adverse Impacts of Transgenic Crops/Foods a Compilation of Scientific References With Abstracts” and from the GMO Free USA website from April 2016 and from 1 Facebook post by GMO Free USA (GMO Free USA 2017). All of these were published before Sanchez 2017 and therefore would have been accessible to the authors.
Results: See Supplementary Table Report 3.
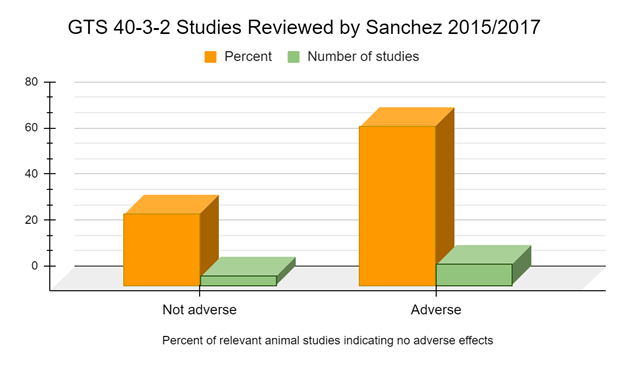
Only 4 animal health studies specifically mentioning, or providing a reference that specifically mentioned, the use of GTS 40-3-2 were used in Sanchez 2015 in which the authors do not report adverse effects (Zhu 2004, Reichert 2012, Bednarek 2013, Świątkiewicz 2013). However, 10 such peer reviewed studies specifically mentioning, or providing a reference that specifically mentioned, the use of GTS 40-3-2 are used in Sanchez 2017 in which the authors report adverse effects or biomarkers indicative of adverse effects (Battistelli 2008, Battistelli 2010, Cisterna 2008, Magaña-Gómez 2008, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2005, Malatesta 2008a, Vecchio 2004). In other words, most studies (71.4%) suggest adverse effects or biomarkers indicative of adverse effects in the animals fed the GM soy GTS 40-3-2 diet.
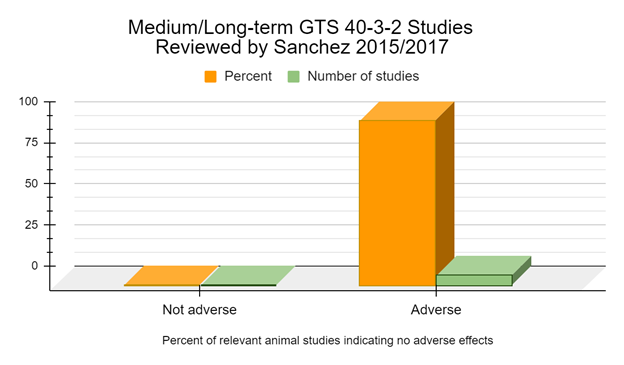
GM foods are generally intended to be consumed for a lifetime by humans and animals of different ages, sexes, and with different medical conditions. Therefore, many health practitioners have concluded that these novel foods should be tested in long-term studies before approval (GMO Free Florida 2022). When we examine the medium and long-term studies, of 6 months feeding duration or longer, 100% of the peer reviewed studies suggest adverse effects or biomarkers indicative of adverse effects from the GM soy GTS 40-3-2 diet (Battistelli 2010, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2008a, Vecchio 2004).
According to Sanchez 2017 when studies “do not indicate which event was evaluated,” this “makes it impossible to replicate the experiments or interpret the results” (Sanchez 2017). However, in order to ensure that these results were not skewed by omitting studies which did not mention the event we also included studies which: 1. mention the soybean having the gene cp4 epsps, 2. Mention the soy used being “Roundup tolerant” or “glyphosate tolerant”, or 3. mention that GM soybeans in general are “Roundup tolerant” or “glyphosate tolerant”. Although soybeans having the gene cp4 epsps could indicate it is event 40-3-2, it is also possible that it could be the experimental line 61-67-1 (Hammond 1996) or another line such as MON-89788-1. The same possibility exists for studies stating the soy is “Roundup tolerant” or “glyphosate tolerant”.
Using the criteria above increases the number of studies not reporting adverse effects to 10 (Zhu 2004, Reichert 2012, Bednarek 2013, Świątkiewicz 2013, Teshima 2000, Sbruzzi 2013, Brake 2004, Sakamoto 2007, Sakamoto 2008, Daleprane 2010). However, 13 peer reviewed studies report adverse effects or biomarkers indicative of adverse effects were observed or that non-GMO soy had a greater health benefit than GM soy (Azevedo 2010, Battistelli 2008, Battistelli 2010, Cisterna 2008, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2005, Malatesta 2008a, Vecchio 2004, Magaña-Gómez 2008, Brasil 2009, Venâncio 2012). In other words, most studies (about 56.5%) report adverse effects or biomarkers indicative of adverse effects from the GM soy diet or that non-GM had a greater health benefit than GM soy. However, of the 10 studies where the authors did not report adverse effects there are examples where other researchers looked at the same data and came to different conclusions (Séralini 2011) or concluded these studies were inadequate to suggest safety (Zdziarski 2014).
When we examine the medium and long-term studies, of 6 months feeding duration or longer, 70% of studies suggest adverse effects or biomarkers indicative of adverse effects from the GM soy diet (Battistelli 2010, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2008a, Vecchio 2004, Brasil 2009, Sakamoto 2007, Sakamoto 2008, Daleprane 2010)
Results from analyzing Sanchez 2015 and 2017, using a consistent standard, references claimed to have been used and above criteria:
It should also be noted that 2 of the studies used by Sanchez 2015, which met the above criteria and did not report adverse effects were in a language other than English (Sakamoto 2007, Sakamoto 2008). Sanchez 2017, however, applied an English language, or Tower of Babel, bias and excluded any studies where adverse effects were observed that were not in English. While an English language bias itself could alter the results of a systematic review, Sanchez took this bias even further and applied favoritism by allowing non-English studies with no adverse effects reported to be included in their overall tally, but excluded any studies where adverse effects were reported that were not in English. Therefore, in order to apply a consistent standard we also included studies from the references claimed to be used by Sanchez 2017 that were not in English.
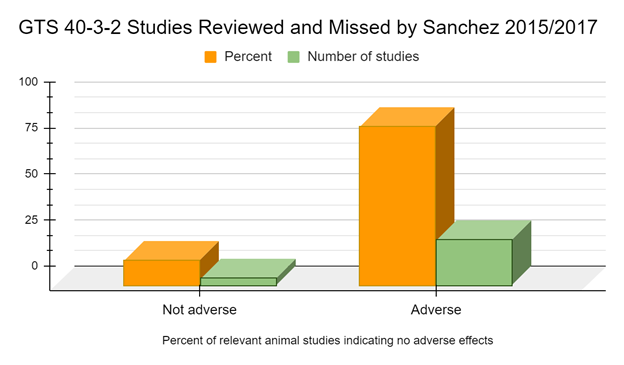
When non-English language studies are included only 4 studies specifically mentioning, or providing a reference that specifically mentioned, the use of GTS 40-3-2 are used in Sanchez 2015 that the authors do not report adverse effects (Zhu 2004, Reichert 2012, Bednarek 2013, Świątkiewicz 2013). However, there are 25 peer reviewed studies specifically mentioning, or providing a reference that specifically mentioned, the use of GTS 40-3-2, from sources claimed to be used in Sanchez 2017, in which adverse effects or biomarkers indicative of adverse effects are reported (Battistelli 2008, Battistelli 2010, Cisterna 2008, Magaña-Gómez 2008, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2005, Malatesta 2008a, Vecchio 2004, Dolaychuk 2013, Ermakova 2008, Ermakova 2009, Gorbach 2012, Gubin-Vakulik 2012, Gubin Vakulik 2013, Gubina-Vakulik 2014, Maligin 2008, Nazarova 2010, Samsonyk 2012, Samsonyk 2013a, Samsonyk 2013b, Samsonyk 2014, Semenov 2014, Zinoviev 2014). In other words, most studies (about 86.2%) suggest adverse effects or biomarkers indicative of adverse effects in the GM soy GTS 40-3-2 diet groups.
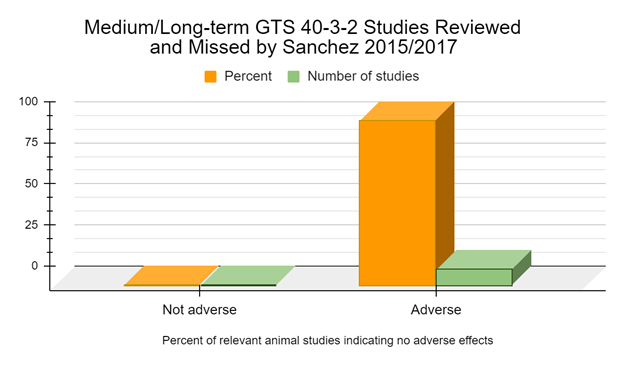
When we examine the medium and long-term studies, of 6 months feeding duration or longer, 100% of studies suggest adverse effects or biomarkers indicative of adverse effects in the GM soy GTS 40-3-2 diet groups (Battistelli 2010, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2008a, Vecchio 2004, Gorbach 2012, Gubin-Vakulik 2012, Gubin-Vakulik 2013, Zinoviev 2014).
Using the criteria above of including studies which: 1. mention the soybean having the gene cp4 epsps, 2. Mention the soy being “Roundup tolerant” or “glyphosate tolerant” or, 3. mentioning that GM soybeans are “Roundup tolerant” or “glyphosate tolerant”, the number of studies not reporting adverse effects were observed is 10 (Zhu 2004, Reichert 2012, Bednarek 2013, Świątkiewicz 2013, Teshima 2000, Sbruzzi 2013, Brake 2004, Sakamoto 2007, Sakamoto 2008, Daleprane 2010). However, 31 peer reviewed studies report that adverse effects or biomarkers indicative of adverse effects were observed or that non-GMO soy had a greater health benefit than GM soy (Azevedo 2010, Battistelli 2008, Battistelli 2010, Cisterna 2008, Magaña-Gómez 2008, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2005, Malatesta 2008a, Vecchio 2004, Brasil 2009, Dolaychuk 2013, Ermakova 2008, Ermakova 2009, Gorbach 2012, Gubin-Vakulik 2012, Gubin Vakulik 2013, Gubina-Vakulik 2014, Maligin 2008, Nazarova 2010, Samsonyk 2012, Samsonyk 2013a, Samsonyk 2013b, Samsonyk 2014, Semenov 2014, Zinoviev 2014, Kulik 2014, Long 2014, Venâncio 2012, Zhou 2012a). In other words, most studies (about 75.6%) suggest adverse effects or biomarkers indicative of adverse effects in the GM soy diet groups.
When we examine the medium and long-term studies, of 6 months feeding duration or longer, then 80% of studies suggest adverse effects or biomarkers indicative of adverse effects in the GM soy diet groups (Battistelli 2010, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2008a, Vecchio 2004, Gorbach 2012, Gubin-Vakulik 2012, Gubin Vakulik 2013, Zinoviev 2014, Brasil 2009, Kulik 2014, Sakamoto 2007, Sakamoto 2008, Daleprane 2010).
Discussion – Glyphosate Tolerant Soy GTS 40-3-2 Systematic Review:
Sanchez 2017 is fundamentally flawed in a number of ways. The authors fail to identify the exact sources used from GMO Free USA, GMWatch and Coalition for a GM Free India making the results of Sanchez 2017 impossible to replicate. The results of our review, however, indicate that when a consistent standard is applied 86.2% of peer reviewed studies indicate adverse effects or biomarkers indicative of adverse effects from the GM soy GTS 40-3-2 diet. Of the medium and long-term studies using the GM soy GTS 40-3-2 diet, 100% indicate adverse effects or biomarkers indicative of adverse effects.
With regards to the studies where adverse effects were reported that were reviewed in Sanchez 2017 the authors claim, “In general terms, all papers analysed here violate at least one of the basic standards for assessment of GM food/feed safety”. For this claim they reference a European Food Safety Authority (EFSA) 2011 report which states, “Internationally agreed test methods described by the OECD (OECD, b) or by the European Commission (EC, 2002) should be used for toxicity testing” (European Food Safety Authority 2011). The Organisation for Economic Co-operation and Development (OECD), as its name implies, was designed to stimulate the economies of the world by progressing world trade.
In the 1970’s during an FDA audit it was determined that 71% of studies done by Industrial Bio-Test Laboratories (IBT) were considered invalid for having “numerous discrepancies between the study conduct and data” (Seaton 2017). Included among these studies were studies done for Monsanto, developers of GM soy GTS 40-3-2, on the herbicide glyphosate, sprayed on GM soy GTS 40-3-2, for which an EPA reviewer stated, “It is also somewhat difficult not to doubt the scientific integrity of a study when the IBT stated that it took specimens from the uteri (of male rabbits) for histopathological examination” (Jackson 2015).
This led Congress to enact Good Laboratory Practice (GLP) Regulations for the FDA (Seaton 2017) which was based on a draft written by G.D. Searle & Company, a laboratory the FDA also determined had numerous problems with regard to scientific integrity and quality control (Baldeshwiler 2003). In this way GLP was written by industry for industry. A remarkably similar GLP document was then adopted by the OECD a few years later (Huntsinger 2008). GLP, however, has not stopped scientific misconduct. For example, in 1991 Craven Laboratories, was determined to have been involved in scientific misconduct in its testing of pesticides during the same time it was hired to conduct studies on glyphosate for Monsanto (Jackson 2015).
OECD guidelines are intended to be the minimal standard for industry to claim no harm, used to try to avoid cases of fraud, and is not the highest standard. For example, the OECD Test Guidelines at the time of the studies reviewed here were not sensitive enough to detect some toxicity (Buonsante 2014). The OECD Test Guidelines are standards for industry to use when conducting studies themselves, they are not the standard required for non-industry studies to be considered relevant. For example, EFSA also states, “the fact that a study may not be conducted in accordance with Good Laboratory Practice (GLP) does not imply that the study is irrelevant” (European Food Safety Authority 2011a).
However, even when examining the 4 studies finding no adverse effects for GM soy GTS 40-3-2 it can be seen they also violate at least one of the OECD standards. Reichert 2012, Bednarek 2013 and Świątkiewicz 2013 all use pigs as subjects and therefore would fall under OECD Test No. 409. This test states, “Three concentrations, at least, should be used” (OECD 1998). However, Reichert 2012 does not specifically mention the concentration(s) used. Bednarek 2013 uses only one concentration for the same group of porkers of 18% when they are younger growers and 14% as older finishing pigs and one concentration for sows of 4% in the low in grower and 14% in high in sow mixture. Świątkiewicz 2013 uses only 1 concentration for the same group of sows of 4% when they are pregnant and 14% when lactating and 26% for their piglet group. The OECD Test No. 409 also states, “The results of this study include: measurements (weighing at least once a week, food/water consumption) and daily (preferably at the same time) and detailed observations (ophtalmological examination, haematology, clinical biochemistry and urinalysis), as well as gross necropsy and histopathology” (OECD 1998). However, the results for several of these parameters are missing from Reichert 2012, Bednarek 2013 and Świątkiewicz 2013. Also, Zhu 2004 fails to adequately report their results (Zdziarski 2014). This makes all of these studies noncompliant with OECD guidelines.
Studies claiming no harm must indeed meet all of the OECD requirements, at an absolute minimum, to make this claim. Yet even then, a claim of no harm would still be suspect as the OECD standards cannot detect some toxicity (Buonsante 2014). Studies which do not meet all of the OECD standards, however, can be used to claim harm. For example, studies that do not make detailed observations of all of the parameters listed by OECD can still determine adverse effects (e.g., an examination of only a single organ can determine if that organ is damaged). However, studies that examine a limited number of parameters, and not all of the parameters listed by OECD, cannot determine that no adverse effects occurred (e.g., an examination of a single organ cannot be used to claim there are no damaged organs in the entire body).
The results of this review indicate that all studies reviewed were published in journals with similar impact factors, and generally did not test for and/or publish isoflavone results. On the other hand, all of the studies claiming no adverse effects suffer from numerous flaws, primarily that a limited number of parameters were tested and short-durations were used. Contrary to the claim made in Sanchez 2017, none of these studies which do not report adverse effects have met the OECD standard and therefore, have not been conducted under a robust study design. On the other hand, the studies which do report adverse effects do not have to comply with the OECD guidelines to adequately report adverse effects.
The results of this review are alarming as it would be expected that most studies would not report adverse effects because of using such a limited number of parameters and short duration. Yet, the large majority of GTS 40-3-2 studies report adverse effects. At a time when science has a reproducibility crisis, (Ioannidis 2005) the fact that many of these studies report very similar adverse effects only further raises concern as these results are often validated by several studies.
Limitations
It should be noted that we limited our inclusion of references cited on the internet by Coalition for a GM Free India to one document and for inclusion of references cited by GMO Free USA to only their website and 1 Facebook post. Therefore, it is possible that we missed studies that reported adverse effects that were posted by Coalition for a GM Free India and GMO Free USA. It should also be noted that even though Sanchez 2017 mentioned using GM Watch as a source, we did not include any articles from GM Watch in our search. GM Watch was established in 1998 and they often post more than once per day (GMWatch Undated). This would have required including thousands of additional articles in our search and, therefore, it is possible that some relevant studies found on the GM Watch website or social media pages were not included in our review. In fact, it is highly likely that we missed some studies reporting adverse effects that met our criteria, since some such studies are listed in the GMO Research database (https://gmoresearch.org/) and a more recent Facebook post by GMO Free USA (GMO Free USA 2021) that were not included in our review (e.g., Long 2013).
Our review does have some other limitations. Although we included all of the references listed as “Animal Health” from Sanchez 2015, it is possible that Sanchez 2015 was not a thorough review of the studies on GM foods. If Sanchez 2015 did not include several studies, this could explain why so few studies mentioning GTS 40-3-2 did not report adverse effects. This is supported by the fact that we identified many animal health studies mentioning GTS 40-3-2 which reported adverse effects that were not listed in Sanchez 2015. However, this could also just indicate that most of the studies using GTS 40-3-2 report adverse effects.
It is also possible that in Sanchez 2015 studies were mislabeled and put in other categories which should have been labeled as “Animal Health”. As we identified in this review, in Sanchez 2015 some studies were mislabeled as “Animal Health” that either had nothing to do with GMOs or should have been labeled as “Animal Nutrition” or another category. Therefore, it is possible that some actual “Animal Health” studies were miscategorized in Sanchez 2015 and were not included in our review.
Another limitation is that we only reviewed studies from 1993 to 2014, so it is also possible that recent GTS 40-3-2 studies, published after 2014, have not reported adverse effects. However, there are also many studies using GTS 40-3-2 which report adverse effects that were published after 2014 that can be found in the GMO Research database (http://gmoresearch.org) and a more recent Facebook post by GMO Free USA (GMO Free USA 2021). Another limitation is that we only reviewed peer reviewed studies to mimic the criteria used in Sanchez 2015. Systematic reviews, however, should include gray literature (National Institutes of Health 2021, Cochrane Collaboration Undated). Some evidence suggests that including unpublished and/or gray literature in systematic reviews may alter the results (Schmucker 2017). However, there are also several studies using GTS 40-3-2 which report adverse effects in the unpublished and/or gray literature as well that can be found on the GMO Research database (http://gmoresearch.org) and a more recent Facebook post by GMO Free USA (GMO Free USA 2021).
Reevaluation of Sanchez 2015/2017
According to Sanchez 2017 only 5% of studies show adverse effects. Yet, no explanation was given for how they came to this conclusion. Therefore, we took a fresh look at Sanchez 2015 and 2017 to try to determine how they came to this conclusion. As our focus is on the health of animals comparable to humans, we looked at the “Animal Health” studies reported by Sanchez 2015 and 2017 limiting our search to in vitro and in vivo studies using rodents, pigs, dogs, non-human primates and humans. Such studies are one of the main lines of evidence used to claim GM foods currently on the market are as safe as conventional foods. We also used studies cited in the Internet by the Coalition for a GM Free India document “Adverse Impacts of Transgenic Crops/Foods a Compilation of Scientific References With Abstracts”, and from the GMO Free USA website from April 2016 and 1 Facebook post from GMO Free USA (GMO Free USA 2017). All of these were published before Sanchez 2017 and therefore would have been accessible to the authors.
Sanchez 2015 included a study in the “Animal Health” section that appears to have nothing to do with GMOs (Kim 2010). Also included was a study which mentions GMOs, but did not test GMOs and instead tested a glyphosate based herbicide (Malatesta 2008b). If studies testing glyphosate based herbicides were intentionally included in Sanchez 2015 then many studies reporting adverse effects found on the GMO Free USA website were missing in Sanchez 2017 (GMO Free USA Glyphosate Studies). Sanchez 2015 also seems to have mislabeled some studies as being “Animal Health” when these studies should have been labeled as “Transgene Degradation” (Aris 2011) or otherwise did not examine health effects (Soria-Guerra 2011, Madduri 2012). As well as studies that looked at nutrient absorption, not health effects, which should have been labeled as “Animal Nutrition” (Shireen 2002, Tso 2002, Molvig 1997). There was also a literature review that should have been excluded (Knudsen 2007). These were all excluded from our reevaluation.
Sanchez 2015 also included several studies using animals not comparable to humans (Zhang 2000a, Humphrey 2002, Glencross 2003, Singh 2003, Shimada 2003, Hemre 2005, Sanden 2005, Shimada 2006a, Sanden 2006, Sung 2006, Koch 2006, Shimada 2006b, Hemre 2007, Sagstad 2007, Bakke‐McKellep 2007, Stumpff 2007, Frøystad 2008, Sagstad 2008, Bondzio 2008, Trabalza-Marinucci 2008, Bakke-McKellep 2008, Sissener 2009, Sissener 2009a, Frøystad‐Sauge 2009, Grisolia 2009a, Sissener 2010, Scholtz 2010, Anilkumar 2010, Brusetti 2011, Sissener 2011, Tripathi 2011, Tripathi 2012, Guertler 2012, Sanden 2013, Gu 2013, Gao 2013, Gao 2014). These studies were excluded from our reevaluation.
Many studies listed by Sanchez 2015 used a purified protein or a item not consumed by humans (Vazquez-Padron 1999, Vázquez 1999, Vazquez-Padron 2000, Vazquez-Padron 2000a, Moreno-Fierros 2000, Moreno-Fierros 2002, Atkinson 2004, Hileman 2006, Shimada 2006, Peng 2007, Onose 2008, Peng 2008, Grisolia 2009, Xu 2009, Verdin‐Terán 2009, Guimaraes 2010, Quemada 2010, Cao 2010, Galbas 2011, Bondzio 2013, Fuchs 1993, Hérouet 2005, Delaney 2007, Herouet-Guicheney 2009, Juberg 2009, Mathesius 2009, Stagg 2012, Dryzga 2007). In Sanchez 2017 studies suggesting adverse effects that used a purified protein were excluded. Yet, in Sanchez 2015 such studies were included. This inconsistent standard distorts the review data. Therefore, we excluded all studies using purified proteins and non-food items.
Many studies listed by Sanchez 2015 as “Animal Health” were in a language other than English (Wang 2000, Sakamoto 2007, Sakamoto 2008, Tutel’ian 2010, Tyshko 2010, Zhuo 2004, Zhuo 2004a, Chen 2004, Li 2004, Li 2004a, Li 2004b, Tutel’ian 2008, Tyshko 2008, Tutel’ian 2009, Tyshko 2009, Li 2010, Tyshko 2011, Hu 2012, Liang 2012). In Sanchez 2017 studies suggesting adverse effects that were not in English were excluded. Yet, in Sanchez 2015 non-English studies were included. This inconsistent standard, once again, distorts the review data. Since there was no purpose mentioned for excluding non-English studies from Sanchez 2017, we included all non-English studies from Sanchez 2015, as well as the non-English studies that should have been included in Sanchez 2017 to form a consistent standard. We also added in English language studies from Sanchez 2017 as well as English language studies that were missed in Sanchez 2017 that were found on the GMO Free USA website (El-Nahas 2011, Marrelli 2013).
We identified 50 studies reporting adverse effects or that the non-GMO food was healthier than the GM food (Fares 1998, Ewen 1999, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Vecchio 2004, Malatesta 2005, Kosieradzka 2005, Seralini 2007, Cisterna 2008, Kılıç 2008, Malatesta 2008a, Finamore 2008, Magaña-Gómez 2008, Brasil 2009, De Vendômois 2009, Battistelli 2010, Azevedo 2010, Venâncio 2012, Seralini 2014, Dolaychuk 2013, Ermakova 2008, Ermakova 2009, Gorbach 2012, Gubin-Vakulik 2012, Gubin Vakulik 2013, Gubina-Vakulik 2014, Maligin 2008, Nazarova 2010, Samsonyk 2012, Samsonyk 2013a, Samsonyk 2013b, Samsonyk 2014, Semenov 2014, Zinoviev 2014, Kulik 2014, Long 2014, Zhou 2012a, Alba 2012, El-Nahas 2011, Marrelli 2013, Oraby 2015, Prescott 2005, Battistelli 2008, Kiliçgün 2013, El-Shamei 2012, Gab-Alla 2012, Abdo 2014, Carman 2013, El-Kholy 2014).
We also identified another 18 studies reporting unintended effects or which the authors otherwise could not conclude safety (Hashimoto 1999, Momma 2000, Kosieradzka 2004, Krzyzowska 2010, Pusztai 1999, Richards 2003, Juśkiewicz 2004, Poulsen 2007, Poulsen 2007a, Schrøder 2007, Kroghsbo 2008, Yonemochi 2010, Li 2010a, Li 2010, Xu 2011, Buzoianu 2012, Walsh 2013, Wang 2013a).
We identified 39 studies not reporting adverse/unintended effects, or where the authors did not specifically state they could not conclude on the safety of the GM food used (Teshima 2000, Wang 2000, Teshima 2002, Zhu 2004, Brake 2004, Brake 2004a, Zdunczyk 2005, Kosieradzka 2005a, Seek Rhee 2005, Sakamoto 2007, Sakamoto 2008, Tsai 2008, Tutel’ian 2008, Tyshko 2008, Fallarero 2009, Domon 2009, Singh 2009, Tutel’ian 2009, Tyshko 2009, Daleprane 2010, Tutel’ian 2010, Tyshko 2010, Stoykova 2011, Yen 2011, Adel-Patient 2011, Tyshko 2011, Buzoianu 2012a, Reichert 2012, Arjó 2012, Buzoianu 2012b, Buzoianu 2012c, Walsh 2012, Langkilde 2012, Buzoianu 2013, Bednarek 2013, Lin 2013, Buzoianu 2013a, Sbruzzi 2013, Tyshko 2014).
We also identified 52 studies not reporting adverse/unintended effects, or where the authors did not specifically state they could not conclude on the safety of the GM food used, with financial or professional conflicts of interest. COIs include: 1. authors that were either developers of GM crops/foods or employees of a company that develops GM crops/foods. 2. study was funded by a company, foundation, etc. that develops or funds the development of GM crops/foods. These include, but are not limited to, every “Animal Health” study listed for events 356043, CV127 soy, 305423, Stearidonic acid-enriched soybean, 1507, 1507 x59122, 59122, 98140, DAS-40278-9, 4114, 73496, high-γ-linolenic acid canola and Y642 lysine maize (Appenzeller 2008, Chukwudebe 2012, Delaney 2008, Hammond 2008, Lemke 2010, MacKenzie 2007, Appenzeller 2009, Malley 2007, He 2008, Appenzeller 2009a, Delaney 2013, Delaney 2014, Wainwright 2003, Liu 2004, He 2009, Chen 2003, Hammond 2004, Hammond 2006, Hammond 2006a, Regina 2006, Doull 2007, Healy 2008, Jaszczak 2008, Harris 2008, MacKenzie 2010, Powell 2010, Qi 2012, Hardisty 2013, Wang 2002, Zhuo 2004, Zhuo 2004a, Chen 2004, Li 2004, Li 2004a, Li 2004b, Zhou 2011, Yuan 2011, Liu 2012, Zhou 2012, Cao 2012, Zhu 2012, Tang 2012, Tang 2012a, Hu 2012, Liang 2012, Liang 2013, Zhu 2013, Wang 2013, Yuan 2013, Wang 2014, Zhang 2014, Song 2014).
Conflicts of interest are often associated with outcomes favorable to the interests of GM seed companies (Guillemaud 2016). Therefore, adverse effects are unlikely to be reported for events where only studies with a COI exist. It should be noted that in several studies funding sources and/or other conflicts of interest were not disclosed. In one study a relationship was found between absence of declaration of funding and outcomes favorable to the interests of GM seed companies (Diels 2011). It is also possible that some of the authors or their funders were developers of GM crops and this was not disclosed. Even when conflict of interest statements declare that GM seed companies were not involved in the study, this may not be accurate as GM seed company employees have already secretly ghostwritten or edited parts of papers without the authors declaring this conflict of interest (Krimsky 2018). Therefore, the number of studies with conflicts of interest could be higher than what is indicated here.
The results of our reevaluation indicate that 63.6% of studies without a conflict of interest report adverse effects, that the non-GMO food was healthier than the GM food, unintended effects were reported, or that the authors otherwise could not conclude safety. As with our review on GTS 40-3-2, and unnamed glyphosate tolerant soy, it should be noted that we limited our inclusion of references cited on the internet by Coalition for a GM Free India to one document and by GMO Free USA to only their website and 1 Facebook post and we did not include any articles from GM Watch in our search. Therefore, it is possible that we missed studies that reported adverse effects that were posted by Coalition for a GM Free India, GMO Free USA and GM Watch. In fact, it is highly likely that we missed some studies reporting adverse effects that met our criteria since some such studies are listed in the GMO Research database (https://gmoresearch.org/) and a more recent Facebook post by GMO Free USA (GMO Free USA 2021) that were not included in our review (e.g., Long 2013).
Inconsistent standard and flaws in assessment of Animal Health Studies:
While Sanchez 2017 does not explain how they came to the conclusion that only 5% of studies using GM foods report adverse effects, it would appear they used the 698 references from Sanchez 2015 which included 18 of the 35 listed as adverse in Sanchez 2017 (Fares 1998, Ewen 1999, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Vecchio 2004, Malatesta 2005, Seralini 2007, Cisterna 2008, Kilic 2008, Malatesta 2008a, Finamore 2008, Magaña‐Gómez 2008, Trabalza-Marinucci 2008, Brasil 2009, De Vendômois 2009, Battistelli 2010, Seralini 2014) leaving an additional 17 studies reporting adverse effects. This added together makes 715 total studies of which Sanchez 2017 identified 35 reporting adverse effects, or about 4.9% reporting adverse effects. There are, however, an abundance of problems with this claim, namely in Sanchez 2017 double standards for inclusion criteria were applied, there were also miscategorized reports and omission of relevant reports.
For example, in Sanchez 2017 a double standard was applied of allowing reports not in English of their choosing, but excluding studies reporting adverse effects that were not in English. Also, the double standard of allowing studies using purified proteins, but excluding any which indicated adverse effects was applied. Sanchez 2015 also has 106 studies listed as “Equivalence” which has little relevance for safety, since one can transform a plant to express a toxin that could kill humans and that plant could still have similar basic vitamin, mineral, etc. content. Nutritional equivalence, by itself, does not equal safety and those studies do not support a claim of safety. For equivalence only a limited number of biologically active substances are considered in isolation and not how they interact with trait-related characteristics (Miyazaki 2019, Benevenuto 2022).
In Sanchez 2015 there is also 111 studies that are listed as “Animal Nutrition” which again does not consider health parameters such as toxicity or carcinogenicity and cannot be used to claim safety. Even if in Sanchez 2017 the authors mistakenly believed such studies were relevant, this would not explain why they omitted studies from the GMO Free USA website, for example, which report nutritional non-equivalence (e.g., Abdo 2013, Lappe 1999, Bøhn 2014). It is only through double standards for inclusion criteria, miscategorized reports, inclusion of irrelevant reports and omission of relevant reports that Sanchez 2017 could come to such an unsubstantiated conclusion that only 5% of studies using GM foods report adverse effects.
As with our review of GTS 40-3-2 studies, all of the “Animal Health” studies not reporting adverse effects suffer from numerous flaws, mostly that a limited number of parameters were tested and the durations were very short. As we previously mentioned, studies that examine a limited number of parameters can determine adverse effects, however, they cannot determine that no adverse effects occurred. Therefore, contrary to the claim made in Sanchez 2017, none of these studies reporting no adverse effects, “have been conducted under a robust study design”.
Of the reviewed studies with no conflicts of interest, the research group including V.A. Tutel’ian has the greatest number of studies reporting no adverse effects with a total of 8 such studies. Yet, in a 2017 review involving V.A. Tutel’ian it was stated that toxicological tests that are only using one mammal and only lasting 90 days are insufficient, and toxicology studies should be extended for the full life span of the test organism and more than one mammal should be used (Tsatsakis 2017). This indicates that even the most prolific independent author of studies indicating no adverse effects still recognizes that these studies by themselves are insufficient to claim safety and further research is necessary. In this same review it states, “Recent claims of consensus over the safety of genetically modified organisms (GMOs) seems to be an artificial and misguided perpetuated construct” (Tsatsakis 2017). Therefore, the evidence is clearly insufficient to claim any GM foods are safe, yet there is an abundance of evidence from animal studies that GTS 40-3-2 is not as safe as its conventional counterparts.
Inconsistent Standard for Conflicts of Interest:
Sanchez 2017 also applies an inconsistent standard for conflict of interest. In Sanchez 2015 conflict of interest is described as, “financial COIs—those that arise when research is fully or partially funded by a party with a stake in the development of GM crops; and also for professional COIs—those that arise when at least one author is affiliated with a company developing GM crops, even if the research is supported through public funding“.
In Sanchez 2017, however, COIs are described as, “Financial COIs arise when research is fully or partially funded by a party with a stake in the development of GM crops or in activities anti-GMO, whereas professional COIs arise when at least one author is affiliated with a company developing GM crops or anti-GMO institutions, even if the research is supported through public funding.”
Even if the standard stated in Sanchez 2017 is used, it was applied selectively. For example it is argued in Sanchez 2017 that J.L. Domingo, author of a review on GM foods (Domingo 2011), had a COI when he was an editor of Food and Chemical Toxicology journal at the time a study by G.E. Séralini (Séralini 2014a) was accepted. In this case Sanchez 2017 argues that because Domingo published a literature review which questioned the lack of safety testing for GM foods that, somehow, implies a COI even though Sanchez 2017 provides no evidence for Domingo having a conflict of interest. By this logic everyone who has ever conducted a study or published a review and concluded in favor of GM foods or crops must also have a conflict of interest for no reason other than their conclusion.
On the other hand, Wallace Hayes was the editor of the journal Food Chemical Toxicology from 2012-2014 and retracted the Séralini study previously mentioned (Séralini 2014). Wallace Hayes was under contract with Monsanto starting in 2012 with a reported fee of $400 per hour for up to $16,000 (Authorization Letter to Consulting Agreement 2012). Yet, this COI was not mentioned in Sanchez 2017. However, Sanchez 2015 lists 8 studies published in Food Chemical Toxicology between 2012 and 2014 which were not considered to have a conflict of interest for this reason (Wang 2014, Zhang 2014, Zhu 2013, Wang 2013, Mishra 2012, Liu 2012, Qi 2012, Zhou 2012). This indicates a double standard was applied for COIs in Sanchez 2017.
In another example, the Federation of Animal Science Societies (FASS) is owned by the Poultry Science Association, the American Dairy Science Association (ADSA), and the American Society of Animal Science (ASAS) according to their website (Federation of Animal Science Societies Undated). Both the ADSA and the ASAS have received funding directly from Monsanto (Animal and Dairy News 2002). Between the years of at least 2002-2014, Gary Hartnell from Monsanto Company has been involved with ADSA as a director and then with FASS on their Scientific Advisory Committee on Biotechnology (Animal and Dairy News 2002, Federation of Animal Science Societies 2014). Monsanto’s Gary Hartnell even served as the president of FASS from 2007 to 2008 (Federation of Animal Science Societies 2008). The FASS publishes the following journals: Journal of Animal Science, Journal of Dairy Science, Poultry Science, Journal of Applied Poultry Research. Studies published in these journals are listed in Sanchez 2015, and 13 studies published in these journals were considered to be without a conflict of interest for this reason in Sanchez 2015 (Buzoianu 2013, Kim 2010, Beagle 2006, Wiedemann 2006, Scholtz 2010, Gao 2012 Gao 2013, Zhang 2000, Chowdhury 2003, Guthrie 2004, Roush 2004, Barriere 2001, Ash 2003). Here is a group with direct funding from, and affiliation with, Monsanto that is not considered a COI by Sanchez 2015.
Another example is the journal Proceedings of the National Academy of Sciences of the United States of America which is listed as the official journal of the National Academy of Sciences (NAS). The NAS received funding from biotechnology companies such as Monsanto (Krimsky 2017). Studies published in these journals are listed in Sanchez 2015 and 7 studies published in these journals were not considered to have a conflict of interest for this reason (Molvig 1997, Schubbert 1997, Catchpole 2005, Kristensen 2005, Regina 2006, Batista 2008, Kogel 2010). It should be noted that this is not an exhaustive list of all the individuals or groups which have involvement with GM seed companies which were not considered a COI in Sanchez 2015.
According to Sanchez 2017, Arpad Pusztai is a recognized opponent of GMOs and therefore any study he conducted or even assisted an author with has a COI. However, Pusztai has published research which states the following, “we conclude that transgenic peas may be used in the diet of mammals, including farm animals, particularly at the moderate levels of dietary inclusion recommended in commercial practice” (Pusztai 1999). This statement, in favor of feeding a GM feed to animals, does not support the claim that Pusztai is a recognized opponent of GMOs in general.
If we were to apply the standard used by Sanchez 2017 consistently, then any individual who has ever made a statement in favor of any GMO has a COI, and any group which has done so is therefore a pro-GMO initiative. Anyone who provides funding to such an individual or group would then be a pro-GMO initiative as well. In this case, every study reporting no adverse effects would have to be considered to have a COI. Therefore, applying such a standard would make all studies have a COI which detracts from actual COIs.
On the other hand, many funders with a stake in the development of GM crops do not appear to have been considered a COI by Sanchez 2015/2017. For example, Bio-oriented Technology Research Advancement Institution of Japan (BRAIN), funds research on the development of GM crops (Ku 1999, Matoba 2001). Yet, animal health studies funded by BRAIN (Hashimoto 1999, Momma 2000) do not seem to be considered as having a COI. The Rockefeller Foundation funds the development of GM crops (Rockefeller Archive Center Undated). Yet, an animal health study funded by the Rockefeller Foundation did not seem to be considered as having a COI (Wang 2002). Many animal health studies appear to have been funded by the Chinese government (Chen 2003, Zhuo 2004, Zhuo 2004a, Chen 2004, Li 2004, Li 2004a, Li 2004b, He 2008, He 2009, Li 2010, Li 2010a, Zhou 2011, Xu 2011, Yuan 2011, Liu 2012, Qi 2012, Zhou 2012, Cao 2012, Zhu 2012, Tang 2012, Tang 2012a, Hu 2012, Liang 2012, Liang 2013, Zhu 2013, Wang 2013, Wang 2013a, Yuan 2013, Wang 2014, Zhang 2014, Song 2014) The Chinese government funds the development of GM crops (Jia 2002) and ChemChina, now one of the largest GM seed companies, is a Chinese state-owned business. Yet, these studies did not seem to have been considered as having a COI for this reason.
Conclusion Sanchez Reviews
The Sanchez 2017 review suffers from numerous flaws. The results cannot be replicated and the inconsistent standards applied make the claims in Sanchez 2017 largely unscientific. When consistent standards are applied the results indicate an alarming number of studies reporting adverse effects.
Nicolia Review
Sanchez 2017 also claims that Nicolia 2014 supports a claim that only 5% of studies using GM foods report adverse effects. Nicolia 2014 concluded, “that the scientific research conducted so far has not detected any significant hazard directly connected with the use of GM crops”. However, they note, “It appears that knowledge on Gene flow and GE food/feed consumption would have benefited from a higher number of publications considering their high impact on both environmental and food/feed risk assessment.” In Nicolia 2014 it is claimed, for GM foods this, “sometimes resulted in animated debate regarding the suitability of the experimental designs, the choice of the statistical methods or the public accessibility of data,” and that this debate, “has frequently been distorted by the media and often used politically and inappropriately in anti-GE crops campaigns”. The results previously presented in our review, however, have demonstrated that all medium and long-term studies using the most tested and most grown GM crop, GTS 40-3-2, report adverse effects or biomarkers indicative of adverse effects.
Criteria:
We applied the same criteria used to review Sanchez 2015 and 2017 to the studies listed in Nicolia 2014 with the timeframe of 2002-2012, the same timeframe used by Nicolia 2014. Studies included were in vivo, using rodents, pigs, dogs, non-human primates and humans, which are more comparable to humans than birds, fish or cattle. Only studies where animals were fed a single GM feed of non-stacked glyphosate tolerant soy GTS 40-3-2, the most analyzed according to Sanchez 2017, as whole feed or processed in a way that humans would consume it were included.
Results from analyzing Nicolia 2014
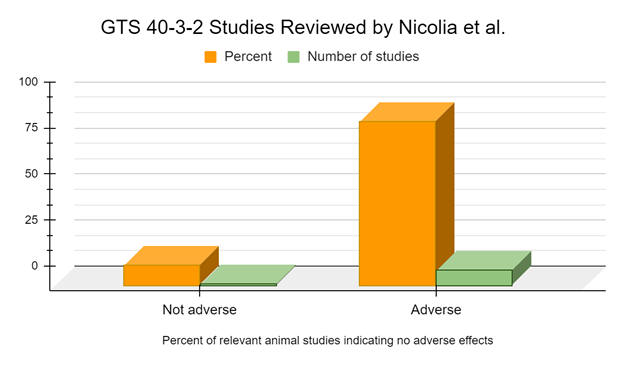
Only 1 relevant animal health study specifically mentioning, or providing a reference that specifically mentioned, the use of GTS 40-3-2 is used in Nicolia 2014 in which the authors do not report adverse effects (Zhu 2004). However, 9 studies specifically mentioning, or providing a reference that specifically mentioned, the use of GTS 40-3-2 are used by Nicolia 2014 in which the authors report adverse effects or biomarkers indicative of adverse effects (Battistelli 2010, Cisterna 2008, Magaña-Gómez 2008, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2005, Malatesta 2008a, Vecchio 2004). This equals 90% of studies suggesting adverse effects were observed.
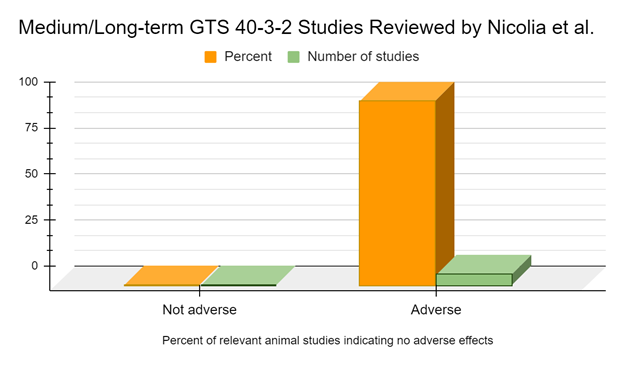
When we examine the medium and long-term studies, of 6 months feeding duration or longer, 100% of studies suggest adverse effects or biomarkers indicative of adverse effects from the GM soy 40-3-2 diet (Battistelli 2010, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2008a, Vecchio 2004).
In order to ensure that these results were not skewed by omitting studies which did not mention the event we also included studies which: 1. mention the soybean having the gene cp4 epsps, 2. Mention soy being “Roundup tolerant” or “glyphosate tolerant” or, 3. mentioning that GM soybeans are “Roundup tolerant” or “glyphosate tolerant”. This resulted in 4 studies not reporting adverse effects (Brake 2004, Zhu 2004, Sakamoto 2008, Daleprane 2010) and 9 studies stating adverse effects or biomarkers indicative of adverse effects were observed (Battistelli 2010, Cisterna 2008, Magaña-Gómez 2008, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2005, Malatesta 2008a, Vecchio 2004) or about 69.2% of studies reporting adverse effects were observed.
Results from analyzing Nicolia 2014 and references missing from review:

However, none of this takes into consideration the numerous studies which Nicolia 2014 missed that appear on the Coalition for a GMO Free India and GMO Free USA internet posts. When this is taken into consideration there is 1 study specifically mentioning the use of GTS 40-3-2 not reporting adverse effects (Zhu 2004). However, 18 studies specifically mentioning the use of GTS 40-3-2 have authors who report adverse effects or biomarkers indicative of adverse effects were observed (Battistelli 2008, Battistelli 2010, Cisterna 2008, Magaña-Gómez 2008, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2005, Malatesta 2008a, Vecchio 2004, Magaña-Gómez 2008, Ermakova 2008, Ermakova 2009, Gorbach 2012, Gubin-Vakulik 2012, Maligin 2008, Nazarova 2010, Samsonyk 2012). This is about 94.7% of studies suggesting adverse effects.
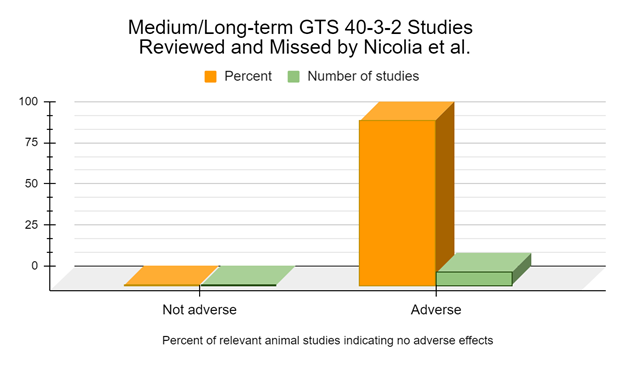
For the medium and long-term studies, of 6 months feeding duration or longer, 100% of the GTS 40-3-2 studies indicate adverse effects or biomarkers indicative of adverse effects from the GM soy GTS 40-3-2 diet (Battistelli 2010, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2008a, Vecchio 2004, Gorbach 2012, Gubin-Vakulik 2012).
In order to ensure that these results were not skewed by omitting studies which did not mention the event we also included studies which: 1. mention the soybean having the gene cp4 epsps, 2. Mention the soy used being “Roundup tolerant” or “glyphosate tolerant” or, 3. mention that GM soybeans are “Roundup tolerant” or “glyphosate tolerant”. This resulted in 4 studies not reporting adverse effects (Brake 2004, Zhu 2004, Sakamoto 2008, Daleprane 2010) and 19 stating adverse effects or biomarkers indicative of adverse effects were observed (Battistelli 2008, Battistelli 2010, Cisterna 2008, Magaña-Gómez 2008, Malatesta 2002a, Malatesta 2002b, Malatesta 2003, Malatesta 2005, Malatesta 2008a, Vecchio 2004, Brasil 2009, Ermakova 2008, Ermakova 2009, Gorbach 2012, Gubin-Vakulik 2012, Maligin 2008, Nazarova 2010, Samsonyk 2012, Zhou 2012a). This indicates about 82.6% report adverse effects.
Discussion
The narrative reviews Nicolia 2014 and Sanchez 2017 suffer from numerous flaws making their conclusions unreliable. We, therefore, conclude that neither Sanchez 2015/2017 or Nicolia 2014 support a claim that only 5% of studies on GM foods observe adverse effects due to a wide variety of errors and omissions. Both Nicolia 2014 and Sanchez 2015 review a large number of GM events which generally have a small number of studies attributed to them. For example, Sanchez 2017 points out that in Sanchez 2015 they reviewed 204 articles that assess animal health for 94 different events. Of those 204 articles, 28 used GTS 40-3-2 and 25 used MON810. This leaves 151 articles that assess 92 different events, or an average of 1.6 articles per event. Results based on a single study are often inaccurate and so this invalidates the claim made by Sanchez 2017 (Ioannidis 2005). Also, as previously mentioned, many of the events only had studies listed that were done by or funded by the companies or researchers that develop GM crops/foods and therefore have a conflict of interest. Studies with a COI most often have outcomes favorable to the interests of the GM seed companies (Guillemaud 2016). Therefore, adverse effects are unlikely to be reported for events where only studies with a COI exist.
On the other hand, there was on average about 17.1 times more studies found for GTS 40-3-2 than for most other events. In this case, Sanchez 2017 should have recognized that events with only 1 or 2 animal health studies did not have robust evidence to conclude safety (Ioannidis 2005), especially when comparing that to the evidence for GTS 40-3-2. Yet, in the reviews by both Nicolia 2014 and Sanchez 2015/2017 they failed to look at the robust evidence for GTS 40-3-2 and instead relied heavily on the 1 or 2 studies done for a large number of events. In fact, many of the events used in these studies are not even on the market, or these studies used animals that are not comparable to humans. This small number of studies for most events indicates how poorly most GM foods have been tested. This is further illustrated in another systematic review where published rat feeding studies examining the digestive tract were only found for 9 of 47 (about 19%) GM foods approved for human and/or animal consumption (Zdziarski 2014). As can be seen from our review, the overwhelming weight of the peer reviewed scientific evidence indicates that GTS 40-3-2 is not likely to be as safe to consume as its conventional counterparts. Our review also indicates the other GM events reviewed have been inadequately tested and any claims that they are as safe as their conventional counterparts must be rejected as they are not supported by sufficient evidence.
Conclusion
Based on the results of this systematic review the claim that all GM foods currently on the market are as safe as their conventional counterparts is not supported by the scientific evidence. Instead, a clear consensus in the scientific literature using the popular GM soy GTS 40-3-2, considered to be the most tested GM food, has emerged. In about 86.2% of all relevant animal feeding studies reviewed, and 100% of the medium and long-term studies, using GTS 40-3-2, adverse effects or biomarkers indicative of adverse effects were reported. This is consistent with the consensus among health experts that GM foods currently on the market cannot presently be considered as safe as their conventional counterparts. This is either due to lack of evidence of safety, or because of evidence that at least some GM foods currently on the market may be unsafe compared to their conventional counterparts. Our systematic reviews find 91.5% of health groups (GMO Free Florida 2022) with statements on GMO safety and no known conflicts of interest, and health professionals in at least 92.9% of surveys (GMO Free Florida 2022a) agree about GM food safety concerns. The majority of health professionals and health students surveyed either believe GM foods have health risks, or are unsure about the safety of GM foods which coincides with the scientific evidence reviewed in this systematic review.
References
Note: If any of the links below are broken, place this before the url: https://web.archive.org/
For example: https://scholar.google.com/scholar?cluster=12344237086176188366
would become https://web.archive.org/https://scholar.google.com/scholar?cluster=12344237086176188366
Abdo, E. M., Barbary, O. M., & Shaltout, O. E. (2013). Chemical Analysis of BT Corn ‘Mon-810: Ajeeb-YG®’and Its Counterpart Non-Bt Corn ‘Ajeeb’. IOSR J Appl Chem, 4(1), 55-60. https://scholar.google.com/scholar?cluster=6817897085759010006
Abdo, E. M., Barbary, O. M., & Shaltout, O. E. S. (2014). Feeding study with Bt corn (MON810: ajeeb YG) on rats: biochemical analysis and liver histopathology. Food and Nutrition Sciences, 2014. https://scholar.google.com/scholar?cluster=17901292899421869801
Adel-Patient, K., Guimaraes, V.D., Paris, A., Drumare, M.F., Ah-Leung, S., Lamourette, P., Nevers, M.C., Canlet, C., Molina, J., Bernard, H. and Créminon, C., (2011). Immunological and metabolomic impacts of administration of Cry1Ab protein and MON 810 maize in mouse. PloS one, 6(1), p.e16346. https://scholar.google.com/scholar?cluster=17172020763221656874
Alba NA, В. Kuz’micheva LV, Е. В. Zinoviev EV (2012). Impact of GM soy on a protein-lipid composition of the blood of animals. International Journal of Applied and Fundamental Research №2.
https://scholar.google.com/scholar?cluster=4286807902548073401
American Association for the Advancement of Science (2012). Statement by the AAAS Board of Directors On Labeling of Genetically Modified Foods. Available from: https://web.archive.org/web/20211207093224/https://www.aaas.org/sites/default/files/AAAS_GM_statement.pdf
Anilkumar, B., Reddy, A.G., Kalakumar, B., Rani, M.U., Anjaneyulu, Y., Raghunandan, T., Reddy, Y.R., Jyothi, K. and Gopi, K.S., (2010). Sero-biochemical studies in sheep fed with Bt cotton plants. Toxicology international, 17(2), p.99. https://scholar.google.com/scholar?cluster=17948152140032415799
Animal and Dairy News (2002). pages 3, 4, 26, 28. Available from: https://web.archive.org/web/20180127130607/http://ucanr.edu/sites/UCCE_LR/files/230578.pdf
Appenzeller, L. M., Munley, S. M., Hoban, D., Sykes, G. P., Malley, L. A., & Delaney, B. (2008). Subchronic feeding study of herbicide–tolerant soybean DP-356Ø43-5 in Sprague–Dawley rats. Food and chemical Toxicology, 46(6), 2201-2213. https://scholar.google.com/scholar?cluster=11613059393753426154
Appenzeller, L. M., Malley, L., MacKenzie, S. A., Hoban, D., & Delaney, B. (2009). Subchronic feeding study with genetically modified stacked trait lepidopteran and coleopteran resistant (DAS-Ø15Ø7-1xDAS-59122-7) maize grain in Sprague-Dawley rats. Food and chemical toxicology, 47(7), 1512-1520. https://scholar.google.com/scholar?cluster=2375196639915838943
Appenzeller, L. M., Munley, S. M., Hoban, D., Sykes, G. P., Malley, L. A., & Delaney, B. (2009a). Subchronic feeding study of grain from herbicide-tolerant maize DP-Ø9814Ø-6 in Sprague-Dawley rats. Food and chemical toxicology, 47(9), 2269-2280. https://scholar.google.com/scholar?cluster=17851307521173409795
Aris, A., & Leblanc, S. (2011). Maternal and fetal exposure to pesticides associated to genetically modified foods in Eastern Townships of Quebec, Canada. Reproductive Toxicology, 31(4), 528-533. https://scholar.google.com/scholar?cluster=17055115353900520704
Arjó, G., Capell, T., Matias‐Guiu, X., Zhu, C., Christou, P., & Piñol, C. (2012). Mice fed on a diet enriched with genetically engineered multivitamin corn show no sub‐acute toxic effects and no sub‐chronic toxicity. Plant biotechnology journal, 10(9), 1026-1034. https://scholar.google.com/scholar?cluster=15497542252204111789
Ash, J., Novak, C., & Scheideler, S. E. (2003). The fate of genetically modified protein from Roundup Ready soybeans in laying hens. Journal of Applied Poultry Research, 12(2), 242-245. https://scholar.google.com/scholar?cluster=6536608337910200040
Atkinson, H. J., Johnston, K. A., & Robbins, M. (2004). Prima facie evidence that a phytocystatin for transgenic plant resistance to nematodes is not a toxic risk in the human diet. The Journal of nutrition, 134(2), 431-434. https://scholar.google.com/scholar?cluster=12533414260648942716
Authorization Letter to Consulting Agreement (2012). Available from: https://web.archive.org/web/20170809141048/http://baumhedlundlaw.com/pdf/monsanto-documents/10-Monsanto-Consulting-Agreement-with-Food-and-Chemical-Toxicology-Editor.pdf
Azevedo, L., Dragano, N. R., Sabino, A. P., Resck, M. C. C., de Lima, P. L. A., & Gouvêa, C. M. (2010). In vivo antimutagenic properties of transgenic and conventional soybeans. Journal of medicinal food, 13(6), 1402-1408. https://scholar.google.com/scholar?cluster=14055039667192285834
Bakke‐McKellep, A. M., Koppang, E. O., Gunnes, G., Sanden, M., Hemre, G. I., Landsverk, T., & Krogdahl, Å. (2007). Histological, digestive, metabolic, hormonal and some immune factor responses in Atlantic salmon, Salmo salar L., fed genetically modified soybeans. Journal of Fish Diseases, 30(2), 65-79. https://scholar.google.com/scholar?cluster=2660454748458928773
Bakke-McKellep, A. M., Sanden, M., Danieli, A., Acierno, R., Hemre, G. I., Maffia, M., & Krogdahl, Å. (2008). Atlantic salmon (Salmo salar L.) parr fed genetically modified soybeans and maize: Histological, digestive, metabolic, and immunological investigations. Research in Veterinary Science, 84(3), 395-408. https://scholar.google.com/scholar?cluster=13909614934273316116
Baldeshwiler, A. M. (2003). History of FDA good laboratory practices. The Quality Assurance Journal: The Quality Assurance Journal for Pharmaceutical, Health and Environmental Professionals, 7(3), 157-161. https://scholar.google.com/scholar?cluster=11968147976664560150
Barriere, Y., Verite, R., Brunschwig, P., Surault, F., & Emile, J. C. (2001). Feeding value of corn silage estimated with sheep and dairy cows is not altered by genetic incorporation of Bt176 resistance to Ostrinia nubilalis. Journal of Dairy Science, 84(8), 1863-1871. https://scholar.google.com/scholar?cluster=7017597760661424127
Batista, R., Saibo, N., Lourenço, T., & Oliveira, M. M. (2008). Microarray analyses reveal that plant mutagenesis may induce more transcriptomic changes than transgene insertion. Proceedings of the National Academy of Sciences, 105(9), 3640-3645. https://scholar.google.com/scholar?cluster=4338415037089355387
Battistelli S., Baldelli B., Malatesta M. (2008). Influence of a GMO-containing diet on pancreatic acinar cells of adult mice: effects of a short-term diet reversion, Microscopie, 10, pp. 36-43. https://scholar.google.com/scholar?cluster=8758600300545104583
Battistelli, S., Citterio, B., Baldelli, B., Parlani, C., & Malatesta, M. (2010). Histochemical and morpho-metrical study of mouse intestine epithelium after a long term diet containing genetically modified soybean. European journal of histochemistry: EJH, 54(3). https://scholar.google.com/scholar?cluster=9126379952876204775&hl=en&as_sdt=0%2C10
Beagle, J.M., Apgar, G.A., Jones, K.L., Griswold, K.E., Radcliffe, J.S., Qiu, X., Lightfoot, D.A. and Iqbal, M.J. (2006). The digestive fate of Escherichia coli glutamate dehydrogenase deoxyribonucleic acid from transgenic corn in diets fed to weanling pigs 1. Journal of animal science, 84(3), pp.597-607. https://scholar.google.com/scholar?cluster=16701656861060570718
Bednarek, D., Dudek, K., Kwiatek, K., Świątkiewicz, M., Świątkiewicz, S., & Strzetelski, J. (2013). Effect of a diet composed of genetically modified feed components on the selected immune parameters in pigs, cattle, and poultry. Bulletin of the Veterinary Institute in Pulawy, 57(2), 209-217. https://scholar.google.com/scholar?cluster=11858751311648421287
Benevenuto, R. F., Venter, H. J., Zanatta, C. B., Nodari, R. O., & Agapito-Tenfen, S. Z. (2022). Alterations in genetically modified crops assessed by omics studies: Systematic review and meta-analysis. Trends in Food Science & Technology. https://scholar.google.com/scholar?cluster=4975695325901713481
Blair, R., & Regenstein, J. M. (2020). GM food and human health. In Genetically Modified and Irradiated Food (pp. 69-98). Academic Press. https://scholar.google.com/scholar?cluster=325556096185215490
Bøhn, T., Cuhra, M., Traavik, T., Sanden, M., Fagan, J., & Primicerio, R. (2014). Compositional differences in soybeans on the market: glyphosate accumulates in Roundup Ready GM soybeans. Food chemistry, 153, 207-215. https://scholar.google.com/scholar?cluster=10458316578824084681
Bondzio, A., Stumpff, F., Schön, J., Martens, H., & Einspanier, R. (2008). Impact of Bacillus thuringiensis toxin Cry1Ab on rumen epithelial cells (REC)–A new in vitro model for safety assessment of recombinant food compounds. Food and Chemical toxicology, 46(6), 1976-1984. https://scholar.google.com/scholar?cluster=2214316219823340376
Bondzio, A., Lodemann, U., Weise, C., & Einspanier, R. (2013). Cry1Ab treatment has no effects on viability of cultured porcine intestinal cells, but triggers Hsp70 expression. PLoS One, 8(7), e67079. https://scholar.google.com/scholar?cluster=1219860354507435246
Brake, D. G., & Evenson, D. P. (2004). A generational study of glyphosate-tolerant soybeans on mouse fetal, postnatal, pubertal and adult testicular development. Food and Chemical Toxicology, 42(1), 29-36. https://scholar.google.com/scholar?cluster=1532669340761942376
Brake, D. G., Thaler, R., & Evenson, D. P. (2004a). Evaluation of Bt (Bacillus thuringiensis) corn on mouse testicular development by dual parameter flow cytometry. Journal of Agricultural and Food Chemistry, 52(7), 2097-2102. https://scholar.google.com/scholar?cluster=10736165784805510765
Brasil, F. B., Soares, L. L., Faria, T. S., Boaventura, G. T., Sampaio, F. J. B., & Ramos, C. F. (2009). The impact of dietary organic and transgenic soy on the reproductive system of female adult rat. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology, 292(4), 587-594. https://scholar.google.com/scholar?cluster=15600228881127269841&hl=en&as_sdt=0%2C10
Brusetti, L., Crotti, E., Tamburini, A., Cittaro, D., Garavaglia, V., Rolli, E., Sorlini, C., Daffonchio, D. and Borin, S., (2011). Influence of transgenic Bt176 and non-transgenic corn silage on the structure of rumen bacterial communities. Annals of microbiology, 61(4), pp.925-930. https://scholar.google.com/scholar?cluster=18098163902643613464
Buonsante, V. A., Muilerman, H., Santos, T., Robinson, C., & Tweedale, A. C. (2014). Risk assessment׳ s insensitive toxicity testing may cause it to fail. Environmental research, 135, 139-147. https://scholar.google.com/scholar?cluster=3216959164954095309
Buzoianu, S.G., Walsh, M.C., Rea, M.C., O’Sullivan, O., Cotter, P.D., Ross, R.P., Gardiner, G.E. and Lawlor, P.G., (2012). High-throughput sequence-based analysis of the intestinal microbiota of weanling pigs fed genetically modified MON810 maize expressing Bacillus thuringiensis Cry1Ab (Bt maize) for 31 days. Applied and environmental microbiology, 78(12), pp.4217-4224. https://scholar.google.com/scholar?cluster=499407115132138891
Buzoianu, S. G., Walsh, M. C., Rea, M. C., Cassidy, J. P., Ross, R. P., Gardiner, G. E., & Lawlor, P. G. (2012a). Effect of feeding genetically modified Bt MON810 maize to∼ 40-day-old pigs for 110 days on growth and health indicators. Animal, 6(10), 1609-1619. https://scholar.google.com/scholar?cluster=10360164568438392726
Buzoianu, S.G., Walsh, M.C., Rea, M.C., O’Donovan, O., Gelencser, E., Ujhelyi, G., Szabo, E., Nagy, A., Ross, R.P., Gardiner, G.E. and Lawlor, P.G., (2012b). Effects of feeding Bt maize to sows during gestation and lactation on maternal and offspring immunity and fate of transgenic material. https://scholar.google.com/scholar?cluster=12748716534057218701
Buzoianu, S.G., Walsh, M.C., Rea, M.C., O’Sullivan, O., Crispie, F., Cotter, P.D., Ross, R.P., Gardiner, G.E. and Lawlor, P.G., (2012c). The effect of feeding Bt MON810 maize to pigs for 110 days on intestinal microbiota. PLoS One, 7(5), p.e33668. https://scholar.google.com/scholar?cluster=592052040955113025
Buzoianu, S.G., Walsh, M.C., Rea, M.C., Cassidy, J.P., Ryan, T.P., Ross, R.P., Gardiner, G.E. and Lawlor, P.G., (2013). Transgenerational effects of feeding genetically modified maize to nulliparous sows and offspring on offspring growth and health. Journal of animal science, 91(1), pp.318-330. https://scholar.google.com/scholar?cluster=2631894927182661511
Buzoianu, S.G., Walsh, M.C., Rea, M.C., Quigley, L., O’Sullivan, O., Cotter, P.D., Ross, R.P., Gardiner, G.E. and Lawlor, P.G., (2013a). Sequence-based analysis of the intestinal microbiota of sows and their offspring fed genetically modified maize expressing a truncated form of Bacillus thuringiensis Cry1Ab protein (Bt maize). Applied and environmental microbiology, 79(24), pp.7735-7744. https://scholar.google.com/scholar?cluster=4637708003708070696
Cao, S., He, X., Xu, W., Ran, W., Liang, L., Luo, Y., Yuan, Y., Zhang, N., Zhou, X. and Huang, K., (2010). Safety assessment of Cry1C protein from genetically modified rice according to the national standards of PR China for a new food resource. Regulatory Toxicology and Pharmacology, 58(3), pp.474-481. https://scholar.google.com/scholar?cluster=17655376343317949649
Cao, S., He, X., Xu, W., Luo, Y., Yuan, Y., Liu, P., Cao, B., Shi, H. and Huang, K., (2012). Safety assessment of transgenic Bacillus thuringiensis rice T1c‐19 in Sprague–Dawley rats from metabonomics and bacterial profile perspectives. IUBMB life, 64(3), pp.242-250. https://scholar.google.com/scholar?cluster=3018583660247983836
Carman, J.A., Vlieger, H.R., Ver Steeg, L.J., Sneller, V.E., Robinson, G.W., Clinch-Jones, C.A., Haynes, J.I. and Edwards, J.W., (2013). A long-term toxicology study on pigs fed a combined genetically modified (GM) soy and GM maize diet. J Org Syst, 8(1), pp.38-54. https://scholar.google.com/scholar?cluster=18354493481142560620
Carvajal, P., Ureña, H., Umaña, J., Sancho, C., Solano, F., Arleo, M., Martínez, C. and Umaña, R., (2017). Detección molecular de secuencias de ADN transgénico en alimentos de consumo humano y animal en Costa Rica. Agronomía Costarricense, 41(1), pp.53-68. https://scholar.google.com/scholar?cluster=16807694581630228173
Catchpole, G.S., Beckmann, M., Enot, D.P., Mondhe, M., Zywicki, B., Taylor, J., Hardy, N., Smith, A., King, R.D., Kell, D.B. and Fiehn, O. (2005). Hierarchical metabolomics demonstrates substantial compositional similarity between genetically modified and conventional potato crops. Proceedings of the National academy of Sciences of the United States of America, 102(40), pp.14458-14462. https://scholar.google.com/scholar?cluster=415508192592748163
Chen, X., Zhuo, Q., Piao, J., & Yang, X. (2004). Immunotoxicologic assessment of transgenetic rice. Wei sheng yan jiu= Journal of hygiene research, 33(1), 77-80. https://scholar.google.com/scholar?cluster=13589019877597456829
Chen, Z.L., Gu, H., Li, Y., Su, Y., Wu, P., Jiang, Z., Ming, X., Tian, J., Pan, N. and Qu, L.J., (2003). Safety assessment for genetically modified sweet pepper and tomato. Toxicology, 188(2-3), pp.297-307. https://scholar.google.com/scholar?cluster=514520258648054058
Chowdhury, E.H., Kuribara, H., Hino, A., Sultana, P., Mikami, O., Shimada, N., Guruge, K.S., Saito, M. and Nakajima, Y. (2003). Detection of corn intrinsic and recombinant DNA fragments and Cry1Ab protein in the gastrointestinal contents of pigs fed genetically modified corn Bt11 1. Journal of animal science, 81(10), pp.2546-2551. https://scholar.google.com/scholar?cluster=10970034828903058968
Chukwudebe, A., Privalle, L., Reed, A., Wandelt, C., Contri, D., Dammann, M., Groeters, S., Kaspers, U., Strauss, V. and van Ravenzwaay, B. (2012). Health and nutritional status of Wistar rats following subchronic exposure to CV127 soybeans. Food and chemical toxicology, 50(3-4), pp.956-971. https://scholar.google.com/scholar?cluster=11611944764121357800
Cisterna, B., Flach, F., Vecchio, L., Barabino, S.M.L., Battistelli, S., Martin, T.E., Malatesta, M. and Biggiogera, M., (2008). Can a genetically-modified organism-containing diet influence embryo development? A preliminary study on pre-implantation mouse embryos. European Journal of Histochemistry, 52(4), pp.263-267. https://scholar.google.com/scholar?cluster=11454636232489383880
Coalition for a GM Free India (2013). Adverse Impacts of Transgenic Crops/Foods: A Compilation of Scientific References With Abstracts. Available from: https://web.archive.org/web/20161121123943/http://re.indiaenvironmentportal.org.in/files/file/Scientific_Papers_Compiled_March_2013_coalition-for-a-gm-free-india.pdf
Cochrane Collaboration (Undated). Chapter 4: Searching for and selecting studies. https://training.cochrane.org/handbook/current/chapter-04#section-4-3-5
Daleprane, J. B., Chagas, M. A., Vellarde, G. C., Ramos, C. F., & Boaventura, G. T. (2010). The Impact of Non‐and Genetically Modified Soybean Diets in Aorta Wall Remodeling. Journal of food science, 75(7). https://scholar.google.com/scholar?cluster=7924315952906456379
Delaney, B., Zhang, J., Carlson, G., Schmidt, J., Stagg, B., Comstock, B., Babb, A., Finlay, C., Cressman, R.F., Ladics, G. and Cogburn, A. (2007). A gene-shuffled glyphosate acetyltransferase protein from Bacillus licheniformis (GAT4601) shows no evidence of allergenicity or toxicity. Toxicological sciences, 102(2), pp.425-432. https://scholar.google.com/scholar?cluster=4322061312841493856
Delaney, B., Appenzeller, L. M., Munley, S. M., Hoban, D., Sykes, G. P., Malley, L. A., & Sanders, C. (2008). Subchronic feeding study of high oleic acid soybeans (event DP-3Ø5423-1) in Sprague–Dawley rats. Food and Chemical Toxicology, 46(12), 3808-3817. https://scholar.google.com/scholar?cluster=9738992118270188555
Delaney, B., Karaman, S., Roper, J., Hoban, D., Sykes, G., Mukerji, P., & Frame, S. R. (2013). Thirteen week rodent feeding study with grain from molecular stacked trait lepidopteran and coleopteran protected (DP-ØØ4114-3) maize. Food and chemical toxicology, 53, 417-427. https://scholar.google.com/scholar?cluster=707888051124772329
Delaney, B., Appenzeller, L. M., Roper, J. M., Mukerji, P., Hoban, D., & Sykes, G. P. (2014). Thirteen week rodent feeding study with processed fractions from herbicide tolerant (DP-Ø73496-4) canola. Food and Chemical Toxicology, 66, 173-184. https://scholar.google.com/scholar?cluster=8360410691341422929
De Vendômois, J. S., Roullier, F., Cellier, D., & Séralini, G. E. (2009). A comparison of the effects of three GM corn varieties on mammalian health. International Journal of Biological Sciences, 5(7), 706. https://scholar.google.com/scholar?cluster=14300992919534485286
Diels, J., Cunha, M., Manaia, C., Sabugosa-Madeira, B., & Silva, M. (2011). Association of financial or professional conflict of interest to research outcomes on health risks or nutritional assessment studies of genetically modified products. Food Policy, 36(2), 197-203. https://scholar.google.com/scholar?cluster=1565868730646714751
Dolaychuk, O. P. , Fedoruk, R. S. (2013). Biological effects of different levels of soybeans conventional and transgenic varieties in the second-generation female rats ration. Animal Biology, 15(2), 30. https://scholar.google.com/scholar?cluster=9016696315544442875
Domingo, J. L., & Bordonaba, J. G. (2011). A literature review on the safety assessment of genetically modified plants. Environment International, 37(4), 734-742. https://scholar.google.com/scholar?cluster=2759829316682760708
Domon, E., Takagi, H., Hirose, S., Sugita, K., Kasahara, S., Ebinuma, H., & Takaiwa, F. (2009). 26-Week oral safety study in macaques for transgenic rice containing major human T-cell epitope peptides from Japanese cedar pollen allergens. Journal of agricultural and food chemistry, 57(12), 5633-5638. https://scholar.google.com/scholar?cluster=17568313993274861411
Dona, A., & Arvanitoyannis, I. S. (2009). Health risks of genetically modified foods. Critical reviews in food science and nutrition, 49(2), 164-175. https://scholar.google.com/scholar?cluster=2941931847779947170&hl=
Doull, J., Gaylor, D., Greim, H. A., Lovell, D. P., Lynch, B., & Munro, I. C. (2007). Report of an Expert Panel on the reanalysis by of a 90-day study conducted by Monsanto in support of the safety of a genetically modified corn variety (MON 863). Food and Chemical Toxicology, 45(11), 2073-2085. https://scholar.google.com/scholar?cluster=13892630407555624768
Dryzga, M. D., Yano, B. L., Andrus, A. K., & Mattsson, J. L. (2007). Evaluation of the safety and nutritional equivalence of a genetically modified cottonseed meal in a 90-day dietary toxicity study in rats. Food and Chemical Toxicology, 45(10), 1994-2004. https://scholar.google.com/scholar?cluster=12875559994144424952
Eckerstorfer, M. F., Heissenberger, A., Reichenbecher, W., Steinbrecher, R. A., & Waßmann, F. (2019). An EU perspective on biosafety considerations for plants developed by genome editing and other new genetic modification techniques (nGMs). Frontiers in bioengineering and biotechnology, 7. https://scholar.google.com/scholar?cluster=12049981105055901807&hl=
El-Kholy, T. A., Hilal, M. A., Al-Abbadi, H. A., Serafi, A. S., Al-Ghamdi, A. K., Sobhy, H. M., & Richardson, J. R. (2014). The effect of extra virgin olive oil and soybean on DNA, cytogenicity and some antioxidant enzymes in rats. Nutrients, 6(6), 2376-2386. https://scholar.google.com/scholar?cluster=3997005353785335281
El-Nahas, A. F.; Mohamed, A. A. A.; Zweel, H. H.; El-Ashwamy, I. M (2011). Hepatorenal and genotoxic effects of genetically modified quail meat in a 90-day dietary toxicity study in mice. International Food Research Journal . Nov, Vol. 18 Issue 4, p1313-1319. 7p. https://scholar.google.com/scholar?cluster=9184382702067203488
Elsanhoty, R. M., Al-Turki, A. I., & Ramadan, M. F. (2013). Prevalence of genetically modified rice, maize, and soy in Saudi food products. Applied biochemistry and biotechnology, 171(4), 883-899. https://scholar.google.com/scholar?cluster=4741578080663475935
El-Shamei, Z. S., Gab-Alla, A. A., Shatta, A. A., Moussa, E. A., & Rayan, A. M. (2012). Histopathological changes in some organs of male rats fed on genetically modified corn (Ajeeb YG). J Am Sci, 8(10), 684-696. https://scholar.google.com/scholar?cluster=16629466726939243714
Ermakova, I.V., Barskov, I.V. (2008). Study of the physiological and morphological parameters in rats and their offspring using a diet containing soybean transgenic EPSPS CP4. Biological sciences. 6. p.19-20. ISSN-1817-6321. Available from: https://web.archive.org/web/20220111210138/https://online.rae.ru/237
Ermakova, I.V. (2009). Influence of soybean gene EPSPS CP4 on the physiological state and reproductive functions of rats in the first two generations Contemporary Problems in Science and Education. Number 5, p.15-20. https://scholar.google.com/scholar?cluster=3445892270525739007
European Food Safety Authority Panel on Genetically Modified Organisms (GMO). (2011). Guidance for risk assessment of food and feed from genetically modified plants. EFSA Journal, 9(5), 2150. https://scholar.google.com/scholar?cluster=13684961949260031996
European Food Safety Authority. (2011a). Submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009. EFSA Journal, 9(2), 2092. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2011.2092
Ewen, S. W., & Pusztai, A. (1999). Effect of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine. The Lancet, 354(9187), 1353-1354. https://scholar.google.com/scholar?cluster=14765610763606486314
Fallarero, A., Ainasoja, M., Sandberg, M., Teeri, T. H., & Vuorela, P. M. (2009). GT1-7 cell-based cytoxicity screening assay on 96-well microplates as a platform for the safety assessment of genetically modified Gerbera hybrida extracts. Drug and chemical toxicology, 32(2), 120-127. https://scholar.google.com/scholar?cluster=8358695257465349415
Fares, N. H., & El-Sayed, A. K. (1998). Fine Structural Changes in the Ileum of Mice Fed on -Endotoxin-Treated Potatoes and Transgenic Potatoes. Natural toxins, 6(6), 219-234. https://scholar.google.com/scholar?cluster=17705777013836847184
Federation of Animal Science Societies (2008). Board of Directors. Available from: https://web.archive.org/web/20080622170836/http://www.fass.org:80/fassboard.asp
Federation of Animal Science Societies (2014). Scientific Advisory Committee on Biotechnology. Available from: https://web.archive.org/web/20140407033413/http://fass.org/sac_biotech.asp
Federation of Animal Science Societies (Undated). Our history and mission. Available from: https://web.archive.org/web/20140407035450/http://www.fass.org/page.asp?pageID=41
Finamore, A., Roselli, M., Britti, S., Monastra, G., Ambra, R., Turrini, A., & Mengheri, E. (2008). Intestinal and peripheral immune response to MON810 maize ingestion in weaning and old mice. Journal of agricultural and food chemistry, 56(23), 11533-11539. https://scholar.google.com/scholar?cluster=12499267478706298503
Frøystad, M. K., Lilleeng, E., Bakke‐McKellep, A. M., Vekterud, K., HEMRE, G. I., & Krogdahl, Å. (2008). Gene expression in distal intestine of Atlantic salmon (Salmo salar L.) fed genetically modified soybean meal. Aquaculture Nutrition, 14(3), 204-214. https://scholar.google.com/scholar?cluster=10793470678751462754
Frøystad‐Saugen, M. K., Lilleeng, E., Bakke‐McKellep, A. M., Vekterud, K., Valen, E. C., HEMRE, G. I., & Krogdahl, Å. (2009). Distal intestinal gene expression in Atlantic salmon (Salmo salar L.) fed genetically modified maize. Aquaculture nutrition, 15(1), 104-115. https://scholar.google.com/scholar?cluster=15317904920815494433
Fuchs, R. L., Ream, J. E., Hammond, B. G., Naylor, M. W., Leimgruber, R. M., & Berberich, S. A. (1993). Safety assessment of the neomycin phosphotransferase II (NPTII) protein. Bio/technology, 11(12), 1543-1547. Available from: https://web.archive.org/web/20220109032841/https://www.researchgate.net/profile/Bruce-Hammond-2/publication/15435436_Safety_Assessment_of_the_Neomycin_Phosphotransferase_II_NPTII_Protein/links/5b8db60f299bf114b7f04c4d/Safety-Assessment-of-the-Neomycin-Phosphotransferase-II-NPTII-Protein.pdf
Gab-Alla, A. A., El-Shamei, Z. S., Shatta, A. A., Moussa, E. A., & Rayan, A. M. (2012). Morphological and biochemical changes in male rats fed on genetically modified corn (Ajeeb YG). J Am Sci, 8(9), 1117-1123. https://scholar.google.com/scholar?cluster=9698337781170883007
Galbas, M., Borys, K., Woźniak, A., & Selwet, M. (2011). Impact of Globulins Derived from Genetically Modified and Conventional Soybean on Swine Lymphocyte Proliferation in Cultures. Annals of Animal Science, 11(4), 497-505. https://scholar.google.com/scholar?cluster=6316035885176722454
Gao, C. Q., Ma, Q. G., Ji, C., Luo, X. G., Tang, H. F., & Wei, Y. M. (2012). Evaluation of the compositional and nutritional values of phytase transgenic corn to conventional corn in roosters. Poultry science, 91(5), 1142-1148. https://scholar.google.com/scholar?cluster=3997810621777272821
Gao, C. Q., Ji, C., Zhao, L. H., Zhang, J. Y., & Ma, Q. G. (2013). Phytase transgenic corn in nutrition of laying hens: Residual phytase activity and phytate phosphorus content in the gastrointestinal tract. Poultry science, 92(11), 2923-2929. https://scholar.google.com/scholar?cluster=3318756889968151952
Gao, C. Q., Ji, C., Zhao, L. H., Zhang, J. Y., & Ma, Q. G. (2013). Phytase transgenic corn in nutrition of laying hens: Residual phytase activity and phytate phosphorus content in the gastrointestinal tract. Poultry science, 92(11), 2923-2929. https://scholar.google.com/scholar?cluster=3318756889968151952
Gao, C., Ma, Q., Zhao, L., Zhang, J., & Ji, C. (2014). Effect of dietary phytase transgenic corn on physiological characteristics and the fate of recombinant plant DNA in laying hens. Asian-Australasian journal of animal sciences, 27(1), 77. https://scholar.google.com/scholar?cluster=9144492659847017011
Glencross, B., Curnow, J., Hawkins, W., Kissil, G. W. M., & Peterson, D. (2003). Evaluation of the feed value of a transgenic strain of the narrow‐leaf lupin (Lupinus angustifolius) in the diet of the marine fish, Pagrus auratus. Aquaculture nutrition, 9(3), 197-206. https://scholar.google.com/scholar?cluster=5751670401080031200
GMO Free Florida (2022). Health Groups. Systematic Reviews. Available from: https://gmofreeflorida.com
GMO Free Florida (2022a). Health Surveys. Systematic Reviews. Available from: https://gmofreeflorida.com
GMO Free USA (2017). Facebook post. Available from: https://www.facebook.com/GMOFreeUSA/posts/1580511311989326:0
GMO Free USA (2021). Facebook post. Available from: https://www.facebook.com/GMOFreeUSA/photos/a.468695639837571/5643611042345979/
GMO Free USA (Undated). Glyphosate Studies. Available from: https://web.archive.org/web/20160402070920/https://gmofreeusa.org/research/glyphosate/glyphosate-studies/
GMO Free USA website as it appears in April 2016. Available from: https://web.archive.org/web/20160403215816/https://gmofreeusa.org/research/gmo-science-research
GMWatch (Undated). About GMWatch. Available from: https://web.archive.org/web/20220103205426/https://www.gmwatch.org/en/main-menu/about
Gorbach, T. V., Kuzmina, I. Yu., Gubina-Vakulik, G. I., & Kolousova, N. G. (2012). Hormonal Regulation of Sexual Function and Ovarian Histological Features in the Experiment With GMO-Soya Use in Food. Tavricheskiy Mediko-Biologicheskiy Vestnik 2012, Volume 15, № 2, Part 2 (58) pages 235-238. https://scholar.google.com/scholar?cluster=8875053064905780580&hl=en&as_sdt=0,10
Grazina, L., Plácido, A., Costa, J., Fernandes, T. J., Oliveira, M. B. P., & Mafra, I. (2017). Tracing two Roundup Ready™ soybean lines (GTS 40-3-2 and MON89788) in foods commercialised in Portugal. Food Control, 73, 1053-1060. https://scholar.google.com/scholar?cluster=15636099947531205933
Grisolia, C. K., Oliveira-Filho, E. C., Ramos, F. R., Lopes, M. C., Muniz, D. H. F., & Monnerat, R. G. (2009). Acute Toxicity and Cytotoxicity of Bacillus Thuringiensis and Bacillus Sphaericus Strains on Fish and Mouse Bone Marrow. Ecotoxicology (London, England), 18(1), 22-26. https://scholar.google.com/scholar?cluster=2829843179953489136
Grisolia, C.K., Oliveira, R., Domingues, I., Oliveira-Filho, E.C., Monerat, R.G. and Soares, A.M., (2009a). Genotoxic evaluation of different δ-endotoxins from Bacillus thuringiensis on zebrafish adults and development in early life stages. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 672(2), pp.119-123. https://scholar.google.com/scholar?cluster=15401180849215998593
Gu, J., Krogdahl, Å., Sissener, N.H., Kortner, T.M., Gelencser, E., Hemre, G.I. and Bakke, A.M., (2013). Effects of oral Bt-maize (MON810) exposure on growth and health parameters in normal and sensitised Atlantic salmon, Salmo salar L. British journal of nutrition, 109(8), pp.1408-1423. https://scholar.google.com/scholar?cluster=7374213628898532699
Gubin-Vakulik, S.A. Denisenko, T.V. Horbach, N.G. Kolousova, T.M. Popova (2012). Morphofunctional State of Adrenal Gland in Female Wistar Rats With Genetically Modified Soy Inclusion in the Diet. Tavricheskiy Mediko-Biologicheskiy Vestnik, Volume 15, № 3, Part 1 (59) pages 85-88. https://scholar.google.com/scholar?cluster=6677984610444828134&hl=en&as_sdt=0%2C10
Gubina-Vakulik, G. I., Gorbach, T. V., Myasoedov, V. V., Kolousova, N. G., & Hopkalov, V. G. (2013a). Metabolic and histological changes in the kidneys of female rats and descendants of the 1st generation when eating genetically modified soybeans. Actual problems of medicine, 22(11 (154)). https://scholar.google.com/scholar?cluster=14620060795757698417
Gubina-Vakulik, G. I., Denisenko, S. A., Gorbach, T. V., Kolousova, N. G., & Andreev, A. V. (2014). Morphofunctional state of the adrenal glands in adult offspring when genetically modified soybeans are introduced into the diet. Experimental and Clinical Medicine. 63 (2), 63-67. https://scholar.google.com/scholar?cluster=901519430211949510
Guertler, P., Brandl, C., Meyer, H. H., & Tichopad, A. (2012). Feeding genetically modified maize (MON810) to dairy cows: comparison of gene expression pattern of markers for apoptosis, inflammation and cell cycle. Journal für Verbraucherschutz und Lebensmittelsicherheit, 7(3), 195-202. https://scholar.google.com/scholar?cluster=7507614196394743799
Guillemaud, T., Lombaert, E., & Bourguet, D. (2016). Conflicts of interest in GM Bt crop efficacy and durability studies. PloS one, 11(12), e0167777. https://scholar.google.com/scholar?cluster=8122887774146003181&hl=en&as_sdt=0%2C10
Guimaraes, L.M., Farias, D.F., Muchagata, R.C.C., de Magalhães, M.Q., Campello, C.C., Rocha, T.L., Vasconcelos, I.M., Carvalho, A.F.U., Mulinari, F. and Grossi-de-Sa, M.F., (2010). Short-term evaluation in growing rats of diet containing Bacillus thuringiensis Cry1Ia12 entomotoxin: nutritional responses and some safety aspects. Journal of biomedicine and biotechnology, 2010. https://scholar.google.com/scholar?cluster=7764333488771371343
Guthrie, T. A., Apgar, G. A., Griswold, K. E., Lindemann, M. D., Radcliffe, J. S., & Jacobson, B. N. (2004). Nutritional value of a corn containing a glutamate dehydrogenase gene for growing pigs1. Journal of animal science, 82(6), 1693-1698. https://scholar.google.com/scholar?cluster=11635196462453711133
Hammond, B. G., & Vicini, J. L. (1996). The feeding value of soybeans fed to rats, chickens, catfish and dairy cattle is not altered by genetic incorporation of glyphosate tolerance. The Journal of nutrition, 126(3), 717. https://scholar.google.com/scholar?cluster=562045050153658015
Hammond, B., Dudek, R., Lemen, J., & Nemeth, M. (2004). Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food and Chemical Toxicology, 42(6), 1003-1014. https://scholar.google.com/scholar?cluster=14777089080972463295
Hammond, B., Lemen, J., Dudek, R., Ward, D., Jiang, C., Nemeth, M., & Burns, J. (2006). Results of a 90-day safety assurance study with rats fed grain from corn rootworm-protected corn. Food and Chemical Toxicology, 44(2), 147-160. https://scholar.google.com/scholar?cluster=10279740427350118277
Hammond, B. G., Dudek, R., Lemen, J. K., & Nemeth, M. A. (2006a). Results of a 90-day safety assurance study with rats fed grain from corn borer-protected corn. Food and Chemical Toxicology, 44(7), 1092-1099. https://scholar.google.com/scholar?cluster=3215761489685910494
Hammond, B. G., Lemen, J. K., Ahmed, G., Miller, K. D., Kirkpatrick, J., & Fleeman, T. (2008). Safety assessment of SDA soybean oil: results of a 28-day gavage study and a 90-day/one generation reproduction feeding study in rats. Regulatory Toxicology and Pharmacology, 52(3), 311-323. https://scholar.google.com/scholar?cluster=599750787140546053
Hardisty, J. F., Banas, D. A., Gopinath, C., Hall, W. C., Hard, G. C., & Takahashi, M. (2013). Spontaneous renal tumors in two rats from a thirteen week rodent feeding study with grain from molecular stacked trait lepidopteran and coleopteran resistant (DP-ØØ4114-3) maize. Food and chemical toxicology, 53, 428-431. https://scholar.google.com/scholar?cluster=8181437543480846985
Harris, W.S., Lemke, S.L., Hansen, S.N., Goldstein, D.A., DiRienzo, M.A., Su, H., Nemeth, M.A., Taylor, M.L., Ahmed, G. and George, C., (2008). Stearidonic acid‐enriched soybean oil increased the omega‐3 index, an emerging cardiovascular risk marker. Lipids, 43(9), pp.805-811. https://scholar.google.com/scholar?cluster=5342175579309284856
Hashimoto, W., Momma, K., Yoon, H.J., Ozawa, S., Ohkawa, Y., Ishige, T., Kito, M., Utsumi, S. and Murata, K., (1999). Safety assessment of transgenic potatoes with soybean glycinin by feeding studies in rats. Bioscience, biotechnology, and biochemistry, 63(11), pp.1942-1946. https://scholar.google.com/scholar?cluster=1578729754424766180
He, X. Y., Huang, K. L., Li, X., Qin, W., Delaney, B., & Luo, Y. B. (2008). Comparison of grain from corn rootworm resistant transgenic DAS-59122-7 maize with non-transgenic maize grain in a 90-day feeding study in Sprague-Dawley rats. Food and Chemical Toxicology, 46(6), 1994-2002. https://scholar.google.com/scholar?cluster=13057592485718314519
He, X.Y., Tang, M.Z., Luo, Y.B., Li, X., Cao, S.S., Yu, J.J., Delaney, B. and Huang, K.L. (2009). A 90-day toxicology study of transgenic lysine-rich maize grain (Y642) in Sprague–Dawley rats. Food and Chemical Toxicology, 47(2), pp.425-432. https://scholar.google.com/scholar?cluster=424852087433197022
Healy, C., Hammond, B., & Kirkpatrick, J. (2008). Results of a 13-week safety assurance study with rats fed grain from corn rootworm-protected, glyphosate-tolerant MON 88017 corn. Food and chemical toxicology, 46(7), 2517-2524. https://scholar.google.com/scholar?cluster=3050489862044385275
Hemre, G. I., Sanden, M., Bakke‐Mckellep, A. M., Sagstad, A., & Krogdahl, Å. (2005). Growth, feed utilization and health of Atlantic salmon Salmo salar L. fed genetically modified compared to non‐modified commercial hybrid soybeans. Aquaculture Nutrition, 11(3), 157-167. https://scholar.google.com/scholar?cluster=10393758277049323749
Hemre, G.I., Sagstad, A., Bakke-Mckellep, A.M., Danieli, A., Acierno, R., Maffia, M., Froystad, M., Krogdahl, A. and Sanden, M., (2007). Suitability of genetically modified soybean meal in rainbow trout diets. Aquaculture Nutrition, 13(3), pp.186-199. https://scholar.google.com/scholar?cluster=5920917054313981490
Hérouet, C., Esdaile, D.J., Mallyon, B.A., Debruyne, E., Schulz, A., Currier, T., Hendrickx, K., van der Klis, R.J. and Rouan, D., (2005). Safety evaluation of the phosphinothricin acetyltransferase proteins encoded by the pat and bar sequences that confer tolerance to glufosinate-ammonium herbicide in transgenic plants. Regulatory Toxicology and Pharmacology, 41(2), pp.134-149. https://scholar.google.com/scholar?cluster=5216773260873748692
Herouet-Guicheney, C., Rouquié, D., Freyssinet, M., Currier, T., Martone, A., Zhou, J., Bates, E.E., Ferullo, J.M., Hendrickx, K. and Rouan, D., (2009). Safety evaluation of the double mutant 5-enol pyruvylshikimate-3-phosphate synthase (2mEPSPS) from maize that confers tolerance to glyphosate herbicide in transgenic plants. Regulatory toxicology and pharmacology, 54(2), pp.143-153. https://scholar.google.com/scholar?cluster=14810473394556277540
Hileman, R. E., Bonner, H. K., Kaempfe, T. A., Hammond, B. G., & Glenn, K. C. (2006). Safety assessment of Cre recombinase. Journal of agricultural and food chemistry, 54(22), 8640-8647. https://scholar.google.com/scholar?cluster=12049615316657043867
Ho, M. W. (2013). The new genetics and natural versus artificial genetic modification. Entropy, 15(11), 4748-4781. https://scholar.google.com/scholar?cluster=11290094311944334911&hl=
Hu, Y., Piao, J., & Yang, X. (2012). Nutritional components and sub-chronic toxicity of genetically modified rice expressing human lactoferrin. Wei sheng yan jiu= Journal of hygiene research, 41(1), 6-12. https://scholar.google.com/scholar?cluster=5039181308158481884
Humphrey, B. D., Huang, N., & Klasing, K. C. (2002). Rice expressing lactoferrin and lysozyme has antibiotic-like properties when fed to chicks. The Journal of nutrition, 132(6), 1214-1218. https://scholar.google.com/scholar?cluster=5047750326459287658
Huntsinger, D. W. (2008). OECD and USA GLP applications. Annali dell”Istituto Superiore di Sanita, 44(4), 403. https://scholar.google.com/scholar?cluster=5934269912143528598
Icahn School of Medicine at Mount Sinai (Undated). Evidence Based Medicine Guide: What’s Best: The Evidence Hierarchy. Available from: https://web.archive.org/web/20211014232703/https://libguides.mssm.edu/ebm/hierarchy
Ioannidis, J. P. (2005). Why most published research findings are false. PLoS medicine, 2(8), e124. https://scholar.google.com/scholar?cluster=15681017780418799273
ISAAA Brief 55 (2019). Executive Summary. Available from: https://www.isaaa.org/resources/publications/briefs/55/executivesummary/default.asp
Jackson, I. (2015) Monsanto Faces Scientific Fraud Claims In Farm Worker Roundup Lawsuits. AboutLawsuits.
https://www.aboutlawsuits.com/roundup-science-fraud-88324/
Jaszczak, K., Kruszewski, M., Baranowski, A., Parada, R., Bartlomiejczyk, T., Zimny, J., & Rosochacki, S. (2008). Micronucleus test and comet assay on mice fed over five generations a diet containing genetically modified triticale. Journal of Animal and Feed Sciences, 17(1), 100. https://scholar.google.com/scholar?cluster=13492727030885877224
Jia, S., & Peng, Y. (2002). GMO biosafety research in China. Environmental Biosafety Research, 1(1), 5-8. https://scholar.google.com/scholar?cluster=16957706380669156394
Juberg, D. R., Herman, R. A., Thomas, J., Brooks, K. J., & Delaney, B. (2009). Acute and repeated dose (28 day) mouse oral toxicology studies with Cry34Ab1 and Cry35Ab1 Bt proteins used in coleopteran resistant DAS-59122-7 corn. Regulatory Toxicology and Pharmacology, 54(2), 154-163. https://scholar.google.com/scholar?cluster=9398105538430790298
Juśkiewicz, J., Zduńczyk, Z., Frejnagel, S., & Fornal, J. (2004). Caecal fermentation in rats fed diets containing transgenic potato tubers. Journal of Animal and Feed Sciences, 13(2), 93-96. https://scholar.google.com/scholar?cluster=10541911538810298183
Kılıç, A., & Akay, M. T. (2008). A three generation study with genetically modified Bt corn in rats: Biochemical and histopathological investigation. Food and Chemical Toxicology, 46(3), 1164-1170. https://scholar.google.com/scholar?cluster=12799667232932860673
Kiliçgün, H., Gürsul, C., Sunar, M. and Gökşen, G. (2013). The Comparative Effects of Genetically Modified Maize and Conventional Maize on Rats. Journal of Clinical and Analytical Medicine. 4(2), 136– 139. Available from: https://web.archive.org/web/20220110232924/https://www.researchgate.net/profile/Hasan-Kilicguen/publication/280830734_The_Comparative_Effects_of_Genetically_Modified_Maize_and_Conventional_Maize_on_Rats/links/5b98fd884585153105801392/The-Comparative-Effects-of-Genetically-Modified-Maize-and-Conventional-Maize-on-Rats.pdf
Kim, D. K., Lillehoj, H. S., Lee, S. H., Jang, S. I., & Bravo, D. (2010). High-throughput gene expression analysis of intestinal intraepithelial lymphocytes after oral feeding of carvacrol, cinnamaldehyde, or Capsicum oleoresin. Poultry science, 89(1), 68-81. https://scholar.google.com/scholar?cluster=9580740196345817819
Knudsen, I., & Poulsen, M. (2007). Comparative safety testing of genetically modified foods in a 90-day rat feeding study design allowing the distinction between primary and secondary effects of the new genetic event. Regulatory Toxicology and Pharmacology, 49(1), 53-62. https://scholar.google.com/scholar?cluster=10154383972989269534
Koch, M., Strobel, E., Tebbe, C. C., Heritage, J., Breves, G., & Huber, K. (2006). Transgenic maize in the presence of ampicillin modifies the metabolic profile and microbial population structure of bovine rumen fluid in vitro. British journal of nutrition, 96(5), 820-829. https://scholar.google.com/scholar?cluster=8132598918614132263
Kogel, K.H., Voll, L.M., Schäfer, P., Jansen, C., Wu, Y., Langen, G., Imani, J., Hofmann, J., Schmiedl, A., Sonnewald, S. and von Wettstein, D. (2010). Transcriptome and metabolome profiling of field-grown transgenic barley lack induced differences but show cultivar-specific variances. Proceedings of the National Academy of Sciences, 107(14), pp.6198-6203. https://scholar.google.com/scholar?cluster=10401362388348453873
Kosieradzka, I., Sawosz, E., Winnicka, A., Kluciński, W., Malepszy, S., Szwacka, M., & Pastuszewska, B. (2004). The effect of transgenic cucumbers expressing thaumatin on selected immunity parameters in rats. Journal of Animal and Feed Sciences, 13(Suppl. 2), 97-100. https://scholar.google.com/scholar?cluster=12514429577628183297
Kosieradzka, I., Sawosz, E., Skomial, J., & Szopa, J. (2005). Transgenic potato tubers with overexpression of 14-3-3 protein in growing rat diets. 1. Selected hormone activities and liver function status. Journal of Animal and Feed Sciences, 14, 545. https://scholar.google.com/scholar?cluster=17668445091470234023
Kosieradzka, I., Sawosz, E., Skomial, J., Szopa, J., Dudkowska, I., & Pastuszewska, B. (2005a). Transgenic potato tubers with overexpression of 14-3-3 protein in growing rat diets. 2. Redox indices in blood and brain. Journal of Animal and Feed Sciences, 14, 549. https://scholar.google.com/scholar?cluster=11414684414709102987
Krimsky S, Schwab T (2017). Conflicts of interest among committee members in the National Academies’ genetically engineered crop study. PLoS ONE 12(2): e0172317. https://scholar.google.com/scholar?cluster=195014334722233715
Krimsky, S., & Gillam, C. (2018). Roundup litigation discovery documents: Implications for public health and journal ethics. Journal of Public Health Policy, 39(3), 318-326. https://scholar.google.com/scholar?cluster=15838292608029521561
Kristensen, C., Morant, M., Olsen, C. E., Ekstrøm, C. T., Galbraith, D. W., Møller, B. L., & Bak, S. (2005). Metabolic engineering of dhurrin in transgenic Arabidopsis plants with marginal inadvertent effects on the metabolome and transcriptome. Proceedings of the National Academy of Sciences of the United States of America, 102(5), 1779-1784. https://scholar.google.com/scholar?cluster=12528386416966962785
Kroghsbo, S., Madsen, C., Poulsen, M., Schrøder, M., Kvist, P.H., Taylor, M., Gatehouse, A., Shu, Q. and Knudsen, I., (2008). Immunotoxicological studies of genetically modified rice expressing PHA-E lectin or Bt toxin in Wistar rats. Toxicology, 245(1-2), pp.24-34. https://scholar.google.com/scholar?cluster=4249317107631220541
Krzyzowska, M., Wincenciak, M., Winnicka, A., Baranowski, A., Jaszczak, K., Zimny, J., & Niemialtowski, M. (2010). The effect of multigenerational diet containing genetically modified triticale on immune system in mice. Pol J Vet Sci, 13(3), 423-30. https://scholar.google.com/scholar?cluster=14710369290711056879
Ku, M.S., Agarie, S., Nomura, M., Fukayama, H., Tsuchida, H., Ono, K., Hirose, S., Toki, S., Miyao, M. and Matsuoka, M., (1999). High-level expression of maize phosphoenolpyruvate carboxylase in transgenic rice plants. Nature biotechnology, 17(1), pp.76-80. https://scholar.google.com/scholar?cluster=2282398674670412055
Kulik Y.M., Hawryluk A.T., Rautskyiene V.T., Khymych V.A. (2014). Morphological changes of liver, kidneys and adrenal glands of experimental animals in the case of long term feeding with Roundup tolerant genetically modified soya. Bulletin morphology. – 2014. – № 1. – P. 149-153. https://scholar.google.com/scholar?cluster=10379054466059095906
Langkilde, S., Schrøder, M., Frank, T., Shepherd, L.V., Conner, S., Davies, H.V., Meyer, O., Danier, J., Rychlik, M., Belknap, W.R. and McCue, K.F., (2012). Compositional and toxicological analysis of a GM potato line with reduced α-solanine content–A 90-day feeding study in the Syrian Golden hamster. Regulatory Toxicology and Pharmacology, 64(1), pp.177-185. https://scholar.google.com/scholar?cluster=2833140240905170079
Lappe, M.A., Bailey, E.B., Childress, C. and Setchell, K.D.R., (1998). Alterations in clinically important phytoestrogens in genetically modified, herbicide-tolerant soybeans. https://scholar.google.com/scholar?cluster=7945642319028934191
Lemke, S. L., Vicini, J. L., Su, H., Goldstein, D. A., Nemeth, M. A., Krul, E. S., & Harris, W. S. (2010). Dietary intake of stearidonic acid–enriched soybean oil increases the omega-3 index: randomized, double-blind clinical study of efficacy and safety–. The American journal of clinical nutrition, 92(4), 766-775. https://scholar.google.com/scholar?cluster=12560405436431638271
Li, M., Piao, J., & Yang, X. (2010). Subchronic toxicity test of genetically modified rice with double antisense starch-branching enzyme gene. Wei sheng yan jiu= Journal of hygiene research, 39(4), 436-9. https://scholar.google.com/scholar?cluster=14492487035034683834
Li, M., Piao, J. H., Tian, Y., Li, W. D., Li, K. J., & Yang, X. G. (2010a). Postprandial glycaemic and insulinaemic responses to GM-resistant starch-enriched rice and the production of fermentation-related H2 in healthy Chinese adults. British journal of nutrition, 103(7), 1029-1034. https://scholar.google.com/scholar?cluster=9317699385343514813
Li, Y., Piao, J., Chen, X., & Zhuo, Q. (2004). Nutrition assessment of transgenic rice. Wei sheng yan jiu= Journal of hygiene research, 33(3), 303-306. https://scholar.google.com/scholar?cluster=12143889460536527762
Li, Y., Piao, J., Zhuo, Q., Chen, X., & Yang, X. (2004a). Subchronic toxicity test of transgenic rice. Wei sheng yan jiu= Journal of hygiene research, 33(5), 575-578. https://scholar.google.com/scholar?cluster=5037148262603231312
Li, Y., Piao, J., Zhuo, Q., Chen, X., Mao, D., Yang, L., & Yang, X. (2004b). Study on the teratogenicity effects of genetically modified rice with Xa21 on rats. Wei sheng yan jiu= Journal of hygiene research, 33(6), 710-712. https://scholar.google.com/scholar?cluster=18388315043522349832
Liang, C.L., Li, Y.N., Zhang, X.P., Song, Y., Wang, W., Fang, J., Cui, W.M. and Jia, X.D., (2012). Immunotoxicologic assessment of genetically modified drought-resistant wheat T349 with GmDREB1. Zhonghua yu Fang yi xue za zhi [Chinese Journal of Preventive Medicine], 46(6), pp.556-560. https://scholar.google.com/scholar?cluster=15550073067847320579
Liang, C. L., Zhang, X. P., Yan, S. O. N. G., & Jia, X. D. (2013). Immunotoxicological evaluation of wheat genetically modified with TaDREB4 gene on BALB/c mice. Biomedical and Environmental Sciences, 26(8), 663-670. https://scholar.google.com/scholar?cluster=4151304659214753573
Lin, H.T., Yen, G.C., Huang, T.T., Chan, L.F., Cheng, Y.H., Wu, J.H., Yeh, S.D., Wang, S.Y. and Liao, J.W., (2013). Toxicity assessment of transgenic papaya ringspot virus of 823-2210 line papaya fruits. Journal of agricultural and food chemistry, 61(7), pp.1585-1596. https://scholar.google.com/scholar?cluster=7874338321647684905
Liu, J. W., DeMichele, S. J., Palombo, J., Chuang, L. T., Hastilow, C., Bobik, E., & Huang, Y. S. (2004). Effect of long-term dietary supplementation of high-gamma-linolenic canola oil versus borage oil on growth, hematology, serum biochemistry, and N-6 fatty acid metabolism in rats. Journal of agricultural and food chemistry, 52(12), 3960-3966. https://scholar.google.com/scholar?cluster=6247277112607714654
Liu, P., He, X., Chen, D., Luo, Y., Cao, S., Song, H., Liu, T., Huang, K. and Xu, W., (2012). A 90-day subchronic feeding study of genetically modified maize expressing Cry1Ac-M protein in Sprague–Dawley rats. Food and chemical toxicology, 50(9), pp.3215-3221. https://scholar.google.com/scholar?cluster=17906540610661380204
Long, W., Wang, H., Li, W., Shen, X., Bai, J., Wang, D., Wang, X., Fan, S. and Zhou, Z., (2013). A Novel Approach for Evaluation of Food Functions and Safety Applied in RR GM Soybeans. Agricultural Biotechnology, 2(3), p.5. https://scholar.google.com/scholar?cluster=2666869993217997151
Long, W., Shen, X., Xiao-Liang, Z., De-Zhi, W., Sai-Jun, F., Xiao-Guang, W., Ze-Wei, Z. (2014). Evaluation on edible safety of genetically modified soy oil by novel system. Journal of Food Safety and Quality Vol. 5 No. 8. Available from: https://web.archive.org/web/20160608165135/http://www.chinafoodj.com/ch/reader/create_pdf.aspx?file_no=20140606003&flag=1&journal_id=spzljcxb
MacKenzie, S.A., Lamb, I., Schmidt, J., Deege, L., Morrisey, M.J., Harper, M., Layton, R.J., Prochaska, L.M., Sanders, C., Locke, M. and Mattsson, J.L. (2007). Thirteen week feeding study with transgenic maize grain containing event DAS-Ø15Ø7-1 in Sprague–Dawley rats. Food and Chemical Toxicology, 45(4), pp.551-562. https://scholar.google.com/scholar?cluster=18116433952280109504
MacKenzie, S. A., Belcher, L. A., Sykes, G. P., Frame, S. R., Mukerji, P., & Gillies, P. J. (2010). Safety assessment of EPA-rich oil produced from yeast: Results of a 90-day subchronic toxicity study. Regulatory Toxicology and Pharmacology, 58(3), 490-500. https://scholar.google.com/scholar?cluster=387941519756619290
Madduri, K.M., Schafer, B.W., Hasler, J.M., Lin, G., Foster, M.L., Embrey, S.K., Sastry-Dent, L., Song, P., Larrinua, I.M., Gachotte, D.J. and Herman, R.A., (2012). Preliminary safety assessment of a membrane-bound delta 9 desaturase candidate protein for transgenic oilseed crops. Food and chemical toxicology, 50(10), pp.3776-3784. https://scholar.google.com/scholar?cluster=7077698810999262498
Magaña‐Gómez, J. A., Cervantes, G. L., Yepiz‐Plascencia, G., & Barca, A. M. C. D. L. (2008). Pancreatic response of rats fed genetically modified soybean. Journal of Applied Toxicology: An International Journal, 28(2), 217-226. https://scholar.google.com/scholar?cluster=6365152937203781580
Malley, L.A., Everds, N.E., Reynolds, J., Mann, P.C., Lamb, I., Rood, T., Schmidt, J., Layton, R.J., Prochaska, L.M., Hinds, M. and Locke, M., (2007). Subchronic feeding study of DAS-59122-7 maize grain in Sprague-Dawley rats. Food and chemical toxicology, 45(7), pp.1277-1292. https://scholar.google.com/scholar?cluster=970483777247523420
Malatesta, M., Caporaloni, C., Gavaudan, S., Rocchi, M. B., Serafini, S., Tiberi, C., & Gazzanelli, G. (2002a). Ultrastructural morphometrical and immunocytochemical analyses of hepatocyte nuclei from mice fed on genetically modified soybean. Cell structure and function, 27(4), 173-180. https://scholar.google.com/scholar?cluster=6849955716031511273
Malatesta, M., Caporaloni, C., Rossi, L., Battistelli, S., Rocchi, M. B., Tonucci, F., & Gazzanelli, G. (2002b). Ultrastructural analysis of pancreatic acinar cells from mice fed on genetically modified soybean. Journal of Anatomy, 201(5), 409-415. https://scholar.google.com/scholar?cluster=4763940144572217832
Malatesta, M., Biggiogera, M., Manuali, E., & Rocchi, M. B. L. (2003). Fine structural analyses of pancreatic acinar cell nuclei from mice fed on genetically modified soybean. European Journal of Histochemistry, 47(4), 385-388. https://scholar.google.com/scholar?cluster=2629293957991316162
Malatesta, M., Baldelli, B., Battistelli, S., Tiberi, C., Manuali, E., & Biggiogera, M. (2005). Reversibility of hepatocyte nuclear modifications in mice fed on genetically modified soybean. European Journal of Histochemistry, 49(3), 237-242. https://scholar.google.com/scholar?cluster=8058884641398811563
Malatesta, M., Boraldi, F., Annovi, G., Baldelli, B., Battistelli, S., Biggiogera, M., & Quaglino, D. (2008a). A long-term study on female mice fed on a genetically modified soybean: effects on liver ageing. Histochemistry and cell biology, 130(5), 967-977. https://scholar.google.com/scholar?cluster=9516067746014349412
Malatesta, M., Perdoni, F., Santin, G., Battistelli, S., Muller, S., & Biggiogera, M. (2008b). Hepatoma tissue culture (HTC) cells as a model for investigating the effects of low concentrations of herbicide on cell structure and function. Toxicology in vitro, 22(8), 1853-1860. https://scholar.google.com/scholar?cluster=13372741498328333888
Maligin, A. G., & Ermakova, I. V. (2008). Soy diet suppresses reproductive function rodents. Modern problems of science and education № 6. (Annex “Biological sciences”). – C. 26. Available from: https://web.archive.org/web/20200323103621/http://online.rae.ru/301
Malley, L.A., Everds, N.E., Reynolds, J., Mann, P.C., Lamb, I., Rood, T., Schmidt, J., Layton, R.J., Prochaska, L.M., Hinds, M. and Locke, M., (2007). Subchronic feeding study of DAS-59122-7 maize grain in Sprague-Dawley rats. Food and chemical toxicology, 45(7), pp.1277-1292. https://scholar.google.com/scholar?cluster=970483777247523420
Marrelli, M., Tudisco, R., Mastellone, V. and Conforti, F., (2013). A comparative study of phytochemical composition of genetically and non-genetically modified soybean (Glycine max L.) and evaluation of antitumor activity. Natural product research, 27(6), pp.574-578. https://scholar.google.com/scholar?cluster=3852762600271964837
Mathesius, C.A., Barnett Jr, J.F., Cressman, R.F., Ding, J., Carpenter, C., Ladics, G.S., Schmidt, J., Layton, R.J., Zhang, J.X.Q., Appenzeller, L.M. and Carlson, G., (2009). Safety assessment of a modified acetolactate synthase protein (GM-HRA) used as a selectable marker in genetically modified soybeans. Regulatory Toxicology and Pharmacology, 55(3), pp.309-320. https://scholar.google.com/scholar?cluster=7280347944104880863
Matoba, N., Doyama, N., Yamada, Y., Maruyama, N., Utsumi, S., & Yoshikawa, M. (2001). Design and production of genetically modified soybean protein with anti-hypertensive activity by incorporating potent analogue of ovokinin (2–7). Febs Letters, 497(1), 50-54. https://scholar.google.com/scholar?cluster=13175554946908671675
Mesnage, R., Agapito-Tenfen, S.Z., Vilperte, V., Renney, G., Ward, M., Séralini, G.E., Nodari, R.O. and Antoniou, M.N., (2016). An integrated multi-omics analysis of the NK603 Roundup-tolerant GM maize reveals metabolism disturbances caused by the transformation process. Scientific reports, 6(1), pp.1-14. https://scholar.google.com/scholar?cluster=6477406195910091532&hl=en&as_sdt=0%2C10
Mishra, A., Gaur, S. N., Singh, B. P., & Arora, N. (2012). In silico assessment of the potential allergenicity of transgenes used for the development of GM food crops. Food and chemical toxicology, 50(5), 1334-1339. https://scholar.google.com/scholar?cluster=1558699854285278781
Miyazaki, J., Bauer-Panskus, A., Bøhn, T., Reichenbecher, W., & Then, C. (2019). Insufficient risk assessment of herbicide-tolerant genetically engineered soybeans intended for import into the EU. Environmental Sciences Europe, 31(1), 1-21. https://scholar.google.com/scholar?cluster=2683890541576867859
Molvig, L., Tabe, L. M., Eggum, B. O., Moore, A. E., Craig, S., Spencer, D., & Higgins, T. J. (1997). Enhanced methionine levels and increased nutritive value of seeds of transgenic lupins (Lupinus angustifolius L.) expressing a sunflower seed albumin gene. Proceedings of the National Academy of Sciences, 94(16), 8393-8398. https://scholar.google.com/scholar?cluster=12759366691816969076
Momma, K., Hashimoto, W., Yoon, H.J., Ozawa, S., Fukuda, Y., Kawai, S., Takaiwa, F., Utsumi, S. and Murata, K., (2000). Safety assessment of rice genetically modified with soybean glycinin by feeding studies on rats. Bioscience, biotechnology, and biochemistry, 64(9), pp.1881-1886. https://scholar.google.com/scholar?cluster=11541879327992563517
Moreno-Fierros, L., García, N., Gutiérrez, R., López-Revilla, R., & Vázquez-Padrón, R. I. (2000). Intranasal, rectal and intraperitoneal immunization with protoxin Cry1Ac from Bacillus thuringiensis induces compartmentalized serum, intestinal, vaginal and pulmonary immune responses in Balb/c mice. Microbes and infection, 2(8), 885-890. https://scholar.google.com/scholar?cluster=17894959323697693279
Moreno-Fierros, L., Pérez-Ordóñez, I., & Palomar-Morales, M. (2002). Slight influence of the estrous cycle stage on the mucosal and systemic specific antibody response induced after vaginal and intraperitoneal immunization with protoxin Cry1Ac from Bacillus thuringiensis in mice. Life sciences, 71(22), 2667-2680. https://scholar.google.com/scholar?cluster=1463595240400942431
National Institutes of Health (2021). Literature Search: Databases and Gray Literature. https://www.nihlibrary.nih.gov/services/systematic-review-service/literature-search-databases-and-gray-literature
Nazarova, A.F., Ermakova, I.V. (2010). Effect of soy diet on reproductive function and testosterone levels in rats and hamsters. Academy Trinitarism, № 77-6567, publ.15788, 12.02. Available from: https://web.archive.org/web/20210225205602/http://www.trinitas.ru/rus/doc/0016/001c/00161613.htm
Nicolia, A., Manzo, A., Veronesi, F. and Rosellini, D. (2014). An overview of the last 10 years of genetically engineered crop safety research. Critical Reviews in Biotechnology, 34, 77–88. Available from: https://scholar.google.com/scholar?cluster=2912430858546690171
Nikolić, Z., Taški-Ajduković, K., Jevtić, A., & Marinković, D. (2009). Detection of GM soybean in food products by simultaneous employment of three pairs of PCR primers. Food Research International, 42(3), 349-352. https://scholar.google.com/scholar?cluster=8822269517886143893
OECD Guidelines for the Testing of Chemicals, Section 4 (1998). Test No. 409: Repeated Dose 90-Day Oral Toxicity Study in Non-Rodents. Available from: http://www.oecd-ilibrary.org/environment/test-no-409-repeated-dose-90-day-oral-toxicity-study-in-non-rodents_9789264070721-en
Onose, J. I., Imai, T., Hasumura, M., Ueda, M., Ozeki, Y., & Hirose, M. (2008). Evaluation of subchronic toxicity of dietary administered Cry1Ab protein from Bacillus thuringiensis var. Kurustaki HD-1 in F344 male rats with chemically induced gastrointestinal impairment. Food and Chemical Toxicology, 46(6), 2184-2189. https://scholar.google.com/scholar?cluster=1805033536965377842
Oraby, H., Kandil, M., Shaffie, N., & Ghaly, I. (2015). Biological impact of feeding rats with a genetically modified-based diet. Turkish Journal of Biology, 39(2), 265-275. https://scholar.google.com/scholar?cluster=12238613498946587067
Poulsen, M., Kroghsbo, S., Schrøder, M., Wilcks, A., Jacobsen, H., Miller, A., Frenzel, T., Danier, J., Rychlik, M., Shu, Q. and Emami, K., (2007). A 90-day safety study in Wistar rats fed genetically modified rice expressing snowdrop lectin Galanthus nivalis (GNA). Food and Chemical Toxicology, 45(3), pp.350-363. https://scholar.google.com/scholar?cluster=17866340406758130730
Poulsen, M., Schrøder, M., Wilcks, A., Kroghsbo, S., Lindecrona, R.H., Miller, A., Frenzel, T., Danier, J., Rychlik, M., Shu, Q. and Emami, K., (2007a). Safety testing of GM-rice expressing PHA-E lectin using a new animal test design. Food and Chemical Toxicology, 45(3), pp.364-377. https://scholar.google.com/scholar?cluster=18290339352000707234
Powell, M., Wheatley, A. O., Omoruyi, F., Asemota, H. N., Williams, N. P., & Tennant, P. F. (2010). Comparative effects of dietary administered transgenic and conventional papaya on selected intestinal parameters in rat models. Transgenic research, 19(3), 511-518. https://scholar.google.com/scholar?cluster=15520108932733088265
Prescott, V.E., Campbell, P.M., Moore, A., Mattes, J., Rothenberg, M.E., Foster, P.S., Higgins, T.J.V. and Hogan, S.P., (2005). Transgenic expression of bean α-amylase inhibitor in peas results in altered structure and immunogenicity. Journal of agricultural and food chemistry, 53(23), pp.9023-9030. https://scholar.google.com/scholar?cluster=8613255224746504374
Pusztai, A., Bardocz, G. G. S., Alonso, R., Chrispeels, M. J., Schroeder, H. E., Tabe, L. M., & Higgins, T. J. V. (1999). Expression of the insecticidal bean α-amylase inhibitor transgene has minimal detrimental effect on the nutritional value of peas fed to rats at 30% of the diet. The Journal of Nutrition, 129(8), 1597-1603. https://scholar.google.com/scholar?cluster=8356039687081780501
Qi, X., He, X., Luo, Y., Li, S., Zou, S., Cao, S., Tang, M., Delaney, B., Xu, W. and Huang, K. (2012). Subchronic feeding study of stacked trait genetically-modified soybean (3Ø5423× 40-3-2) in Sprague–Dawley rats. Food and chemical toxicology, 50(9), pp.3256-3263. https://scholar.google.com/scholar?cluster=9067977151193983036
Quemada, H., Zarka, K., Pett, W., Bothma, G., Felcher, K., Mirendil, H., Koch, M., Brink, J. and Douches, D., (2010). Safety evaluations of the Cry1Ia1 protein found in the transgenic potato ‘SpuntaG2’. Journal of the American Society for Horticultural Science, 135(4), pp.325-332. https://scholar.google.com/scholar?cluster=7009871960645457373
Rang, A., Linke, B., & Jansen, B. (2005). Detection of RNA variants transcribed from the transgene in Roundup Ready soybean. European Food Research and Technology, 220(3-4), 438-443. https://scholar.google.com/scholar?cluster=2477275647936867897&hl=
Regina, A., Bird, A., Topping, D., Bowden, S., Freeman, J., Barsby, T., Kosar-Hashemi, B., Li, Z., Rahman, S. and Morell, M. (2006). High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proceedings of the National Academy of Sciences of the United States of America, 103(10), pp.3546-3551. https://scholar.google.com/scholar?cluster=2384289212770520280
Reichert, M., Kozaczyński, W., Karpińska, T.A., Bocian, Ł., Jasik, A., Kycko, A., Świątkiewicz, M., Świątkiewicz, S., Furgał-Dierżuk, I., Arczewska-Włosek, A. and Strzetelski, J. (2012). Histopathology of internal organs of farm animals fed genetically modified corn and soybean meal. Bulletin of the Veterinary Institute in Pulawy, 56(4), pp.617-622. https://scholar.google.com/scholar?cluster=3051340787344556572
Richards, H. A., Han, C. T., Hopkins, R. G., Failla, M. L., Ward, W. W., & Stewart Jr, C. N. (2003). Safety assessment of recombinant green fluorescent protein orally administered to weaned rats. The Journal of nutrition, 133(6), 1909-1912. https://scholar.google.com/scholar?cluster=6537642230342403281
Rockefeller Archive Center (Undated). Biotechnology. Available from: https://web.archive.org/web/20211105204659/https://rockfound.rockarch.org/biotechnology
Rosculete, E., Bonciu, E., Rosculete, C. A., & Teleanu, E. (2018). Detection and Quantification of Genetically Modified Soybean in Some Food and Feed Products. A Case Study on Products Available on Romanian Market. Sustainability (2071-1050), 10(5). https://scholar.google.com/scholar?cluster=2216190139052397662
Roush, W. B., & Tozer, P. R. (2004). The power of tests for bioequivalence in feed experiments with poultry 1. Journal of animal science, 82(13_suppl), E110-E118. https://scholar.google.com/scholar?cluster=8497857140294888578
Ryś, P., Władysiuk, M., Skrzekowska-Baran, I., & Małecki, M. T. (2009). Review articles, systematic reviews and meta-analyses: which can be trusted?. Polskie Archiwum Medycyny Wewnętrznej, 119(3), 148-156. https://scholar.google.com/scholar?cluster=16974704546224762536
Sagstad, A., Sanden, M., Haugland, Ø., Hansen, A. C., Olsvik, P. A., & Hemre, G. I. (2007). Evaluation of stress‐and immune‐response biomarkers in Atlantic salmon, Salmo salar L., fed different levels of genetically modified maize (Bt maize), compared with its near‐isogenic parental line and a commercial suprex maize. Journal of fish diseases, 30(4), 201-212. https://scholar.google.com/scholar?cluster=3430971087790332823
Sagstad, A., Sanden, M., Krogdahl, Å., Bakke‐McKellep, A. M., Frøystad, M., & Hemre, G. I. (2008). Organs development, gene expression and health of Atlantic salmon (Salmo salar L.) fed genetically modified soybeans compared to the near‐isogenic non‐modified parental line. Aquaculture Nutrition, 14(6), 556-572. Available from: https://web.archive.org/web/20220116034918/https://www.researchgate.net/publication/229986207_Organs_development_gene_expression_and_health_of_Atlantic_salmon_Salmo_salar_L_fed_genetically_modified_soybeans_compared_to_the_near-isogenic_non-modified_parental_line
Sakamoto, Y., Tada, Y., Fukumori, N., Tayama, K., Ando, H., Takahashi, H., Kubo, Y., Nagasawa, A., Yano, N., Yuzawa, K. and Ogata, A. (2007). A 52-week feeding study of genetically modified soybeans in F344 rats. Shokuhin eiseigaku zasshi. Journal of the Food Hygienic Society of Japan, 48(3), pp.41-50. https://scholar.google.com/scholar?cluster=4437628980933218775
Sakamoto, Y., Tada, Y., Fukumori, N., Tayama, K., Ando, H., Takahashi, H., Kubo, Y., Nagasawa, A., Yano, N., Yuzawa, K. and Ogata, A. (2008). A 104-week feeding study of genetically modified soybeans in F344 rats. Shokuhin eiseigaku zasshi. Journal of the Food Hygienic Society of Japan, 49(4), pp.272-282. https://scholar.google.com/scholar?cluster=923560158218660800
Sakr, J., Mallah, N., Chalak, L., & Abou-Sleymane, G. (2014). First comprehensive GMOs testing in Lebanon: Screening, identification and quantification of GM soybean imports. Food Control, 36(1), 146-152. https://scholar.google.com/scholar?cluster=10887147218699988239
Samsonyk, I., Kocumbas, G.I, Levuckuj, T.R. (2012). Hematological and biochemical indices of blood of rats of the first generation fed GMO soy. Scientific Messenger of Lviv National University of Veterinary Medicine and Biotechnologies. Volume 14, number 2 (52) Part 1, 2012 Pages 156-160. https://scholar.google.com/scholar?cluster=4567081511624726846
Samsonyk, I., Stronskyi, U. (2013a). Biochemical indices of blood of rats for three generations which were fed with GMO soy. Scientific Messenger of Lviv National University of Veterinary Medicine and Biotechnologies. Volume 15 number 3 (57) Part 2, 2013 Pages 279-282. https://scholar.google.com/scholar?cluster=1412112706563189908
Samsonyk, I. (2013b). Structural and functional state of the gastrointestinal tract of rats of the first generation of feeding traditional and genetically modified soybeans. Scientific and Technical Bulletin of the Institute of Animal Biology and State research control institute of veterinary preparations and feed additives. Vol. 14, № 3-4 Pages 238-243. https://scholar.google.com/scholar?cluster=9411142391252588545
Samsonyk, I., Kotcumbas, G.I. (2014). Ultrastructural characteristic of rats liver of the third generation with the influence of genetically modified and traditional soybean. Animal Biology. 2014, т. 16, № 2. https://scholar.google.com/scholar?cluster=14778756796902192149
Sánchez, M.A. (2015). Conflict of interests and evidence base for GM crops food/feed safety research. Nature Biotechnology, 33, 135–137. https://scholar.google.com/scholar?cluster=3047213763081414325
Sánchez, M.A. and Parrott, W.A. (2017). Characterization of scientific studies usually cited as evidence of adverse effects of GM food/feed. Plant Biotechnology Journal., 15, 1227–1234. https://scholar.google.com/scholar?cluster=10770760028361898301
Sanden, M., Berntssen, M. H. G., Krogdahl, Å., Hemre, G. I., & Bakke‐McKellep, A. M. (2005). An examination of the intestinal tract of Atlantic salmon, Salmo salar L., parr fed different varieties of soy and maize. Journal of Fish Diseases, 28(6), 317-330. https://scholar.google.com/scholar?cluster=7289600730646545131
Sanden, M., Krogdahl, Å., Bakke‐Mckellep, A. M., Buddington, R. K., & Hemre, G. I. (2006). Growth performance and organ development in Atlantic salmon, Salmo salar L. parr fed genetically modified (GM) soybean and maize. Aquaculture Nutrition, 12(1), 1-14. https://scholar.google.com/scholar?cluster=4517297920166733323
Sanden, M., Ornsrud, R., Sissener, N. H., Jorgensen, S., Gu, J., Bakke, A. M., & Hemre, G. I. (2013). Cross-generational feeding of Bt (Bacillus thuringiensis)-maize to zebrafish (Danio rerio) showed no adverse effects on the parental or offspring generations. British journal of nutrition, 110(12), 2222-2233. https://scholar.google.com/scholar?cluster=12899571701353932999
Sbruzzi, F. A., Venâncio, V. D. P., Resck, M. C. C., Brigagão, M. R. P. L., & Azevedo, L. (2013). Transgenic and conventional Brazilian soybeans don’t cause or prevent preneoplastic colon lesions or oxidative stress in a 90-day in vivo study. Revista de Nutrição, 26(4), 443-453. https://scholar.google.com/scholar?cluster=429898219989223927
Schmucker, C.M., Blümle, A., Schell, L.K., Schwarzer, G., Oeller, P., Cabrera, L., von Elm, E., Briel, M., Meerpohl, J.J. and OPEN consortium (2017). Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research. PloS one, 12(4), p.e0176210. https://scholar.google.com/scholar?cluster=493661355510357499
Scholtz, N. D., Halle, I., Dänicke, S., Hartmann, G., Zur, B., & Sauerwein, H. (2010). Effects of an active immunization on the immune response of laying Japanese quail (Coturnix coturnix japonica) fed with or without genetically modified Bacillus thuringiensis-maize. Poultry science, 89(6), 1122-1128. https://scholar.google.com/scholar?cluster=12777274749262812756
Schrøder, M., Poulsen, M., Wilcks, A., Kroghsbo, S., Miller, A., Frenzel, T., Danier, J., Rychlik, M., Emami, K., Gatehouse, A. and Shu, Q., (2007). A 90-day safety study of genetically modified rice expressing Cry1Ab protein (Bacillus thuringiensis toxin) in Wistar rats. Food and Chemical Toxicology, 45(3), pp.339-349. https://scholar.google.com/scholar?cluster=8377183865473808575
Schubbert, R., Renz, D., Schmitz, B., & Doerfler, W. (1997). Foreign (M13) DNA ingested by mice reaches peripheral leukocytes, spleen, and liver via the intestinal wall mucosa and can be covalently linked to mouse DNA. Proceedings of the National Academy of Sciences, 94(3), 961-966. https://scholar.google.com/scholar?cluster=1493477396657257738
Seaton, M. (2017). An Update on FDA’s Good Laboratory Practice (GLP) for Nonclinical Laboratory Studies Proposed Rule. https://www.toxicology.org/groups/ss/rsess/doc/2017SOTWebinar_with_notesRSESS_Seaton.pdf
Seek Rhee, G., Hyun Cho, D., Hyuck Won, Y., Hyun Seok, J., Sun Kim, S., Jun Kwack, S., Da Lee, R., Yeong Chae, S., Woo Kim, J., Mu Lee, B. and Lea Park, K., (2005). Multigeneration reproductive and developmental toxicity study of bar gene inserted into genetically modified potato on rats. Journal of Toxicology and Environmental Health, Part A, 68(23-24), pp.2263-2276. https://scholar.google.com/scholar?cluster=15111052090727623487
Semenov, S. O., Bindyug, O. A., Zinoviev, S. G., Basova, L. V., & Semenov, E. S. (2014). The intensity of growth and reproducibility of pigs under the conditions of consumption of GM soybeans. Pig breeding,(64), 143-152. https://scholar.google.com/scholar?cluster=18292221252220823913
Séralini, G. E., Cellier, D., & de Vendomois, J. S. (2007). New analysis of a rat feeding study with a genetically modified maize reveals signs of hepatorenal toxicity. Archives of environmental contamination and toxicology, 52(4), 596-602. https://scholar.google.com/scholar?cluster=7414683643956816990
Séralini, G. E., Mesnage, R., Clair, E., Gress, S., De Vendômois, J. S., & Cellier, D. (2011). Genetically modified crops safety assessments: present limits and possible improvements. Environmental Sciences Europe, 23(1), 10. https://scholar.google.com/scholar?cluster=11024172273198315451
Séralini, G.E., Clair, E., Mesnage, R., Gress, S., Defarge, N., Malatesta, M., Hennequin, D. and de Vendômois, J.S. (2014). Republished study: long-term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Environmental Sciences Europe, 26(1), p.14. https://scholar.google.com/scholar?cluster=11278277407020805608
Séralini, G. E., Mesnage, R., Defarge, N., & de Vendômois, J. S. (2014a). Conflicts of interests, confidentiality and censorship in health risk assessment: the example of an herbicide and a GMO. Environmental Sciences Europe, 26(1), 13. https://scholar.google.com/scholar?cluster=16392527655744288082
Shimada, N., Kim, Y. S., Miyamoto, K., Yoshioka, M., & Murata, H. (2003). Effects of Bacillus thuringiensis Cry1Ab toxin on mammalian cells. Journal of Veterinary Medical Science, 65(2), 187-191. https://scholar.google.com/scholar?cluster=15839195282126934909
Shimada, N., Miyamoto, K., Kanda, K., & Murata, H. (2006). Bacillus thuringiensis insecticidal crylab toxin does not affect the membrane integrity of the mammalian intestinal epithelial cells: an in vitro study. In Vitro Cellular & Developmental Biology-Animal, 42(1-2), 45-49. https://scholar.google.com/scholar?cluster=168355912214475478
Shimada, N., Miyamoto, K., Kanda, K., & Murata, H. (2006a). Binding of Cry1Ab toxin, a Bacillus thuringiensis insecticidal toxin, to proteins of the bovine intestinal epithelial cell: An in vitro study. Applied Entomology and Zoology, 41(2), 295-301. https://scholar.google.com/scholar?cluster=3151523750939365697
Shimada, N., Murata, H., Mikami, O., Yoshioka, M., Guruge, K.S., Yamanaka, N., Nakajima, Y. and Miyazaki, S., (2006). Effects of feeding calves genetically modified corn Bt11: a clinico-biochemical study. Journal of veterinary medical science, 68(10), pp.1113-1115. https://scholar.google.com/scholar?cluster=18427180527689490349
Shireen, K. F., Pace, R. D., Egnin, M., & Prakash, C. S. (2002). Bioavailability of calcium from sweet potato and soy flour supplemented diets in hamsters. Journal of Environmental Science and Health, Part B, 37(6), 637-645. https://scholar.google.com/scholar?cluster=11126105794880521038
Singh, A. K., Praveen, S., Singh, B. P., Varma, A., & Arora, N. (2009). Safety assessment of leaf curl virus resistant tomato developed using viral derived sequences. Transgenic research, 18(6), 877. https://scholar.google.com/scholar?cluster=12484515940864913157
Singh, M., Tiwari, D. P., Kumar, A., & Kumar, M. R. (2003). Effect of feeding transgenic cottonseed vis-a-vis non-transgenic cottonseed on haematobiochemical constituents in lactating Murrah buffaloes. Asian-australasian journal of animal sciences, 16(12), 1732-1737. https://scholar.google.com/scholar?cluster=14075342300163832390
Sissener, N.H., Bakke, A.M., Gu, J., Penn, M.H., Eie, E., Krogdahl, Å., Sanden, M. and Hemre, G.I., (2009). An assessment of organ and intestinal histomorphology and cellular stress response in Atlantic salmon (Salmo salar L.) fed genetically modified Roundup Ready® soy. Aquaculture, 298(1-2), pp.101-110. https://scholar.google.com/scholar?cluster=112504390395535487
Sissener, N. H., Sanden, M., Bakke, A. M., Krogdahl, Å., & Hemre, G. I. (2009a). A long term trial with Atlantic salmon (Salmo salar L.) fed genetically modified soy; focusing general health and performance before, during and after the parr–smolt transformation. Aquaculture, 294(1-2), 108-117. https://scholar.google.com/scholar?cluster=16542104433932819795
Sissener, N. H., Martin, S. A., Cash, P., Hevrøy, E. M., Sanden, M., & Hemre, G. I. (2010). Proteomic profiling of liver from Atlantic salmon (Salmo salar) fed genetically modified soy compared to the near-isogenic non-GM line. Marine Biotechnology, 12(3), 273-281. https://scholar.google.com/scholar?cluster=656602567638190833
Sissener, N.H., Hemre, G.I., Lall, S.P., Sagstad, A., Petersen, K., Williams, J., Rohloff, J. and Sanden, M., (2011). Are apparent negative effects of feeding GM MON810 maize to Atlantic salmon, Salmo salar, caused by confounding factors?. British journal of nutrition, 106(1), pp.42-56. https://scholar.google.com/scholar?cluster=5831453955870214045
Soga, K., Kimata, S., Narushima, J., Sato, S., Sato, E., Mano, J., Takabatake, R., Kitta, K., Kawakami, H., Akiyama, H. and Kondo, K., (2020). Development and Testing of an Individual Kernel Detection System for Genetically Modified Soybean Events in Non-identity-preserved Soybean Samples. Biological and Pharmaceutical Bulletin, 43(8), pp.1259-1266. https://scholar.google.com/scholar?cluster=32752627106373717
Song, Y., Liang, C., Wang, W., Fang, J., Sun, N., Jia, X., & Li, N. (2014). Immunotoxicological evaluation of corn genetically modified with Bacillus thuringiensis Cry1Ah gene by a 30-day feeding study in BALB/c mice. PloS one, 9(2), e78566. https://scholar.google.com/scholar?cluster=975649235717636205
Soria-Guerra, R. E., Rosales-Mendoza, S., Moreno-Fierros, L., López-Revilla, R., & Alpuche-Solís, Á. G. (2011). Oral immunogenicity of tomato-derived sDPT polypeptide containing Corynebacterium diphtheriae, Bordetella pertussis and Clostridium tetani exotoxin epitopes. Plant cell reports, 30(3), 417-424. https://scholar.google.com/scholar?cluster=13403024435799182543
Stagg, N. J., Thomas, J., Herman, R. A., & Juberg, D. R. (2012). Acute and 28-day repeated dose toxicology studies in mice with aryloxyalkanoate dioxygenase (AAD-1) protein expressed in 2, 4-D tolerant DAS-40278-9 maize. Regulatory Toxicology and Pharmacology, 62(2), 363-370. https://scholar.google.com/scholar?cluster=16930855540253918995
Stoykova, P., Radkova, M., Stoeva-Popova, P., Atanasov, N., Chassovnikarova, T., Wang, X., Iantcheva, A., Vlahova, M. and Atanassov, A., (2011). Expression of the human acidic fibroblast growth factor in transgenic tomato and safety assessment of transgenic lines. Biotechnology & Biotechnological Equipment, 25(1), pp.2187-2196. https://scholar.google.com/scholar?cluster=9192001061140571626
Stumpff, F., Bondzio, A., Einspanier, R., & Martens, H. (2007). Effects of the Bacillus thuringiensis toxin Cry1Ab on membrane currents of isolated cells of the ruminal epithelium. Journal of Membrane Biology, 219(1), 37-47. https://scholar.google.com/scholar?cluster=3208507638608177870
Sung, H. G., Min, D. M., Kim, D. K., Li, D. Y., Kim, H. J., Upadhaya, S. D., & Ha, J. K. (2006). Influence of transgenic corn on the in vitro rumen microbial fermentation. Asian-australasian journal of animal sciences, 19(12), 1761-1768. https://scholar.google.com/scholar?cluster=13149124681724370147
Świątkiewicz, M., Bednarek, D., Markowski, J., Hanczakowska, E. and Kwiatek, K., (2013). Effect of feeding genetically modified maize and soybean meal to sows on their reproductive traits, haematological indices and offspring performance. Bull Vet Inst Pulawy, 57, pp.413-418. https://scholar.google.com/scholar?cluster=2174378075136354012
Tang, X., Han, F., Zhao, K., Xu, Y., Wu, X., Wang, J., Jiang, L. and Shi, W., (2012). A 90-day dietary toxicity study of genetically modified rice T1C-1 expressing Cry1C protein in Sprague Dawley rats. PLoS One, 7(12), p.e52507. https://scholar.google.com/scholar?cluster=13969500938770751051
Tang, M., Xie, T., Cheng, W., Qian, L., Yang, S., Yang, D., Cui, W. and Li, K., (2012a). A 90-day safety study of genetically modified rice expressing rhIGF-1 protein in C57BL/6J rats. Transgenic research, 21(3), pp.499-510. https://scholar.google.com/scholar?cluster=10800592772342058649
Teshima, R., Akiyama, H., Okunuki, H., Sakushima, J., Goda, Y., Onodera, H., Sawada, J. and Toyoda, M. (2000). Effect of GM and non-GM soybeans on the immune system of BN rats and B10A mice. Shokuhin Eiseigaku Zasshi= Journal of the Food Hygienic Society of Japan, 41(3), pp.188-193. https://scholar.google.com/scholar?cluster=4417718153878416446
Teshima, R., Watanabe, T., Okunuki, H., Isuzugawa, K., Akiyama, H., Onodera, H., Imai, T., Toyoda, M. and Sawada, J.I., (2002). Effect of Subchronic Feeding of Genetically Modified Corn (CBH351) on Immune System in BN Rats and B10A Mice. Shokuhin eiseigaku zasshi. Journal of the Food Hygienic Society of Japan, 43(5), pp.273-279. https://scholar.google.com/scholar?cluster=14196313649879007297
Trabalza-Marinucci, M., Brandi, G., Rondini, C., Avellini, L., Giammarini, C., Costarelli, S., Acuti, G., Orlandi, C., Filippini, G., Chiaradia, E. and Malatesta, M., (2008). A three-year longitudinal study on the effects of a diet containing genetically modified Bt176 maize on the health status and performance of sheep. Livestock Science, 113(2-3), pp.178-190. https://scholar.google.com/scholar?cluster=7641416471640727997
Tripathi, M. K., Mondal, D., Somvanshi, R., & Karim, S. A. (2011). Haematology, blood biochemistry and tissue histopathology of lambs maintained on diets containing an insect controlling protein (Cry1Ac) in Bt‐cottonseed. Journal of animal physiology and animal nutrition, 95(5), 545-555. https://scholar.google.com/scholar?cluster=16165346363661753854
Tripathi, M. K., Mondal, D., Raghuvansi, S. K. S., & Karim, S. A. (2012). Effect of Bt-cottonseed meal feeding on intake, growth, nutrient utilization, serum cholesterol, immunological status, organ weight and slaughtering performance of growing lambs. Animal Nutrition and Feed Technology, 12(2), 165-178. https://scholar.google.com/scholar?cluster=8339577672748643204
Tsai, C. C., Lai, C. H., Yang, C. S., Lin, C. K., & Tsen, H. Y. (2008). Toxicological evaluation of transgenic rice flour with an Escherichia coli phytase gene appA by sub‐chronic feeding study in Wistar rats. Journal of the Science of Food and Agriculture, 88(3), 382-388. https://scholar.google.com/scholar?cluster=8977074130483248993
Tsatsakis, A.M., Nawaz, M.A., Kouretas, D., Balias, G., Savolainen, K., Tutelyan, V.A., Golokhvast, K.S., Lee, J.D., Yang, S.H. and Chung, G., (2017). Environmental impacts of genetically modified plants: a review. Environmental research, 156, pp.818-833. https://scholar.google.com/scholar?cluster=11940553478694921031
Tso, P., Ding, K., DeMichele, S., & Huang, Y. S. (2002). Intestinal absorption and lymphatic transport of a high γ-linolenic acid canola oil in lymph fistula Sprague-Dawley rats. The Journal of nutrition, 132(2), 218-221. https://scholar.google.com/scholar?cluster=11086203362109132725
Tutel’ian, V.A., Gapparov, M.M., Avren’eva, L.I., Aksiuk, I.N., Guseva, G.V., L’vova, L.S., Saprykin, V.P., Tyshko, N.V. and Chernysheva, O.N., (2008). Medical and biological safety assessment of genetically modified maize event MON 88017. Report 1. Toxicologo-hygienic examinations. Voprosy pitaniia, 77(5), pp.4-12. https://scholar.google.com/scholar?cluster=9349272260819520114
Tutel’ian, V.A., Gapparov, M.M., Avren’eva, L.I., Aksiuk, I.N., Guseva, G.V., Kravchenko, L.V., L’vova, L.S., Saprykin, V.P., Tyshko, N.V. and Chernysheva, O.N., (2009). Medical and biological safety assessment of genetically modified maize strain MIR604. Voprosy pitaniia, 78(2), pp.24-32. https://scholar.google.com/scholar?cluster=4264902416443936524
Tutel’ian, V.A., Gapparov, M.G., Avren’eva, L.I., Guseva, G.V., Zhminchenko, V.M., Kravchenko, L.V., Pashorina, V.A., Saprykin, V.P., Seliaskin, K.E. and Tyshko, N.V., (2010). Medical and biological safety assessment of genetically modified soybean event MON 89788. Report 1. Toxicologo-hygienic examinations. Voprosy pitaniia, 79(3), pp.4-12. https://scholar.google.com/scholar?cluster=13052392063348535402
Tyshko, N.V., Britsina, M.V., Gmoshinskiĭ, I.V., Zhanataev, A.K., Zakharova, N.S., Zorin, S.N., Mazo, V.K. and Semenov, B.F., (2008). Medical and biological safety assessment of genetically modified maize event MON 88017. Report 2. Genotoxicologic, immunologic and allergologic examinations. Voprosy pitaniia, 77(5), pp.13-17. https://scholar.google.com/scholar?cluster=2543541053260496
Tyshko, N.V., Britsina, M.V., Gmoshinskiĭ, I.V., Zhanataev, A.K., Zakharova, N.S., Zorin, S.N., Mazo, V.K., Ozeretskovskaia, M.N. and Semenov, B.F., (2009). Medical and biological safety assessment of genetically modified maize event MIR604. Voprosy pitaniia, 78(2), pp.33-38. https://scholar.google.com/scholar?cluster=11729661326846224762
Tyshko, N.V., Britsina, M.V., Gmoshinskiĭ, I.V., Zakharova, N.S., Zorin, S.N., Mazo, V.K., Ozeretskovskaia, M.N. and Seliaskin, K.E. (2010). Medical and biological safety assessment of genetically modified soybean event MON 89788. Report 2. Genotoxicologic, immunologic and allergologic examinations. Voprosy pitaniia, 79(3), pp.13-17. https://scholar.google.com/scholar?cluster=12184472892866889340
Tyshko, N. V., Zhminchenko, V. M., Pashorina, V. A., Seliaskin, K. E., Saprykin, V. P., Utembaeva, N. T., & Tutel’ian, V. A. (2011). Assessment of the impact of GMO of plant origin on rat progeny development in 3 generations. Voprosy pitaniia, 80(1), 14-28. https://scholar.google.com/scholar?cluster=6119352827113876933
Tyshko, N. V., Zhminchenko, V. M., Selyaskin, K. E., Pashorina, V. A., Utembaeva, N. T., & Tutelyan, V. A. (2014). Assessment of the impact of genetically modified LibertyLink® maize on reproductive function and progeny development of Wistar rats in three generations. Toxicology Reports, 1, 330-340. https://scholar.google.com/scholar?cluster=5249993870753874181
Ujhelyi, G., Vajda, B., Béki, E., Neszlényi, K., Jakab, J., Jánosi, A., Némedi, E. and Gelencsér, É. (2008). Surveying the RR soy content of commercially available food products in Hungary. Food Control, 19(10), pp.967-973. https://scholar.google.com/scholar?cluster=702737316551639565
UTHealth (2020). Systematic Review Resources: Systematic Reviews vs Other Types of Reviews. Available from: https://web.archive.org/web/20210517033849/https://libguides.sph.uth.tmc.edu/c.php?g=543382&p=5370369
Vazquez-Padron, R. I., Moreno-Fierros, L., Neri-Bazán, L., Gustavo, A., & Lopez-Revilla, R. (1999). Intragastric and intraperitoneal administration of Cry1Ac protoxin from Bacillus thuringiensis induces systemic and mucosal antibody responses in mice. Life Sciences, 64(21), 1897-1912. https://scholar.google.com/scholar?cluster=7490918033041759196
Vázquez, R. I., Moreno-Fierros, L., Neri-Bazán, L., De La Riva, G. A., & López-Revilla, R. (1999). Bacillus thuringiensis Cry1Ac protoxin is a potent systemic and mucosal adjuvant. Scandinavian Journal of Immunology, 49(6), 578-584. https://scholar.google.com/scholar?cluster=6629652979937855211
Vázquez-Padrón, R.I., Gonzáles-Cabrera, J., García-Tovar, C., Neri-Bazan, L., Lopéz-Revilla, R., Hernández, M., Moreno-Fierro, L. and Gustavo, A., (2000). Cry1Ac protoxin from Bacillus thuringiensis sp. kurstaki HD73 binds to surface proteins in the mouse small intestine. Biochemical and biophysical research communications, 271(1), pp.54-58. https://scholar.google.com/scholar?cluster=3794219287740494927
Vazquez-Padron, R. I., Moreno-Fierros, L., Neri-Bazan, L., Martinez-Gil, A. F., De-la-Riva, G. A., & Lopez-Revilla, R. (2000a). Characterization of the mucosal and systemic immune response induced by Cry1Ac protein from Bacillus thuringiensis HD 73 in mice. Brazilian Journal of Medical and Biological Research, 33(2), 147-155. https://scholar.google.com/scholar?cluster=7057036577304956841
Vecchio, L., Cisterna, B., Malatesta, M., Martin, T. E., & Biggiogera, M. (2004). Ultrastructural analysis of testes from mice fed on genetically modified soybean. European Journal of Histochemistry, 449-454. https://scholar.google.com/scholar?cluster=7770490040815646318
Venâncio, V. P., Silva, J. P. L., Almeida, A. A., Brigagão, M. R., & Azevedo, L. (2012). Conventional (MG-BR46 Conquista) and transgenic (BRS Valiosa RR) soybeans have no mutagenic effects and may protect against induced-DNA damage in vivo. Nutrition and cancer, 64(5), 725-731. https://scholar.google.com/scholar?cluster=15367464989316591896
Verdin‐Terán, S. L., Vilches‐Flores, A., & Moreno‐Fierros, L. (2009). Immunization with Cry1Ac from Bacillus thuringiensis increases intestinal IgG response and induces the expression of FcRn in the intestinal epithelium of adult mice. Scandinavian journal of immunology, 70(6), 596-607. https://scholar.google.com/scholar?cluster=13212296403522460373
Viljoen, C. D., Dajee, B. K., & Botha, G. M. (2006). Detection of GMO in food products in South Africa: Implications of GMO labelling. African journal of biotechnology, 5(2), 73-82. https://scholar.google.com/scholar?cluster=9784766851110468271
Wainwright, P. E., Huang, Y. S., DeMichele, S. J., Xing, H., Liu, J. W., Chuang, L. T., & Biederman, J. (2003). Effects of high-γ-linolenic acid canola oil compared with borage oil on reproduction, growth, and brain and behavioral development in mice. Lipids, 38(2), 171-178. https://scholar.google.com/scholar?cluster=13735636681702178038
Walsh, M.C., Buzoianu, S.G., Rea, M.C., O’Donovan, O., Gelencser, E., Ujhelyi, G., Ross, R.P., Gardiner, G.E. and Lawlor, P.G., (2012). Effects of feeding Bt MON810 maize to pigs for 110 days on peripheral immune response and digestive fate of the cry1Ab gene and truncated Bt toxin. PloS one, 7(5), p.e36141. https://scholar.google.com/scholar?cluster=6976815268532009230
Walsh, M. C., Buzoianu, S. G., Gardiner, G. E., Rea, M. C., O’Donovan, O., Ross, R. P., & Lawlor, P. G. (2013). Effects of feeding Bt MON810 maize to sows during first gestation and lactation on maternal and offspring health indicators. British journal of nutrition, 109(5), 873-881. https://scholar.google.com/scholar?cluster=17548848060345692781
Wang, E. H., Yu, Z., Hu, J., & Xu, H. B. (2013). Effects of 90-day feeding of transgenic Bt rice TT51 on the reproductive system in male rats. Food and chemical toxicology, 62, 390-396. https://scholar.google.com/scholar?cluster=17911008106004363879
Wang, E. H., Yu, Z., Hu, J., Jia, X. D., & Xu, H. B. (2014). A two-generation reproduction study with transgenic Bt rice TT51 in Wistar rats. Food and chemical toxicology, 65, 312-320. https://scholar.google.com/scholar?cluster=889652075343368250
Wang, Y., Lai, W., Chen, J., & Mei, S. (2000). Toxicity of anti-herbicide gene (BAR) transgenic rice. Wei sheng yan jiu= Journal of hygiene research, 29(3), 141-142. https://scholar.google.com/scholar?cluster=7924582307399464287
Wang, Y., Wei, B., Tian, Y., Wang, Z., Tian, Y., Tan, S., Dong, S. and Song, Q., (2013a). Evaluation of the potential effect of transgenic rice expressing Cry1Ab on the hematology and enzyme activity in organs of female Swiss rats. PloS One, 8(11), p.e80424. https://scholar.google.com/scholar?cluster=3596890604141954394
Wang, Z. H., Wang, Y., Cui, H. R., Xia, Y. W., Altosaar, I., & Shu, Q. Y. (2002). Toxicological evaluation of transgenic rice flour with a synthetic cry1Ab gene from Bacillus thuringiensis. Journal of the Science of Food and Agriculture, 82(7), 738-744. Available from: https://web.archive.org/web/20220116012121/https://www.researchgate.net/publication/229569274_Toxicological_evaluation_of_transgenic_rice_flour_with_a_synthetic_cry1Ab_gene_from_Bacillus_thuringiensis
Wiedemann, S., Lutz, B., Kurtz, H., Schwarz, F. J., & Albrecht, C. (2006). In situ studies on the time-dependent degradation of recombinant corn DNA and protein in the bovine rumen1. Journal of Animal Science, 84(1), 135-144. https://scholar.google.com/scholar?cluster=16868600521741842447
Wilson, A. K., Latham, J. R., & Steinbrecher, R. A. (2006). Transformation-induced mutations in transgenic plants: analysis and biosafety implications. Biotechnology and Genetic Engineering Reviews, 23(1), 209-238. https://scholar.google.com/scholar?cluster=12219212651561893601&hl=
Xu, W., Cao, S., He, X., Luo, Y., Guo, X., Yuan, Y., & Huang, K. (2009). Safety assessment of Cry1Ab/Ac fusion protein. Food and Chemical Toxicology, 47(7), 1459-1465. https://scholar.google.com/scholar?cluster=4885863598995673820
Xu, W., Li, L., Lu, J., Luo, Y., Shang, Y., & Huang, K. (2011). Analysis of Caecal Microbiota in Rats Fed with Genetically Modified Rice by Real‐Time Quantitative PCR. Journal of food science, 76(1), M88-M93. https://scholar.google.com/scholar?cluster=10327696037589414712
Yen, G.C., Lin, H.T., Cheng, Y.H., Lin, Y.J., Chang, S.C., Yeh, S.D., Chan, Y.C., Chung, Y.C. and Liao, J.W., (2011). Food safety evaluation of papaya fruits resistant to papaya ring spot virus. Journal of Food and Drug Analysis, 19(3), pp.269-280. https://scholar.google.com/scholar?cluster=6732685738037162632
Yonemochi, C., Suga, K., Harada, C., & Hanazumi, M. (2010). Tevaluation of transgenic event CBH 351 (StarLink) corn in pig. Animal science journal, 81(1), 94-101. https://scholar.google.com/scholar?cluster=10784891139379984241
Yuan, Y., Xu, W., Luo, Y., Liu, H., Lu, J., Su, C., & Huang, K. (2011). Effects of genetically modified T2A‐1 rice on faecal microflora of rats during 90 day supplementation. Journal of the Science of Food and Agriculture, 91(11), 2066-2072. https://scholar.google.com/scholar?cluster=5741081650041061267
Yuan, Y., Xu, W., He, X., Liu, H., Cao, S., Qi, X., Huang, K. and Luo, Y., (2013). Effects of genetically modified T2A-1 rice on the GI health of rats after 90-day supplement. Scientific reports, 3(1), pp.1-9. https://scholar.google.com/scholar?cluster=499996459423274314
Zdunczyk, Z., Juskiewicz, J., Fornal, J., Mazur-Gonkowska, B., Koncicki, A., Flis, B., Zimnoch-Guzowska, E. and Zagorski-Ostoja, W., (2005). Biological response of rat fed diets with high tuber content of conventionally bred and transgenic potato resistant to necrotic strain of potato virus (PVYN). Part II. Caecal metabolism, serum enzymes and indices of non-specific defence of rats. Food Control, 16(8), pp.767-772. https://scholar.google.com/scholar?cluster=15504910147209915524
Zdziarski, I. M., Edwards, J. W., Carman, J. A., & Haynes, J. I. (2014). GM crops and the rat digestive tract: A critical review. Environment international,73, 423-433. https://scholar.google.com/scholar?cluster=7766678220266402155
Zhang, M., Zhuo, Q., Tian, Y., Piao, J., & Yang, X. (2014). Long-term toxicity study on transgenic rice with Cry1Ac and sck genes. Food and Chemical Toxicology, 63, 76-83. https://scholar.google.com/scholar?cluster=7635410286110749719
Zhang, Z. B., Kornegay, E. T., Radcliffe, J. S., Wilson, J. H., & Veit, H. P. (2000). Comparison of phytase from genetically engineered Aspergillus and canola in weanling pig diets. Journal of Animal Science, 78(11), 2868-2878. https://scholar.google.com/scholar?cluster=7765290083647242215&hl=
Zhang, Z. B., Kornegay, E. T., Radcliffe, J. S., Denbow, D. M., Veit, H. P., & Larsen, C. T. (2000a). Comparison of genetically engineered microbial and plant phytase for young broilers. Poultry Science, 79(5), 709-717. https://scholar.google.com/scholar?cluster=1418530389492375901
Zhou, X.H., Dong, Y., Xiao, X., Wang, Y., Xu, Y., Xu, B., Shi, W.D., Zhang, Y., Zhu, L.J. and Liu, Q.Q., (2011). A 90-day toxicology study of high-amylose transgenic rice grain in Sprague–Dawley rats. Food and chemical toxicology, 49(12), pp.3112-3118. https://scholar.google.com/scholar?cluster=7246603816234940268
Zhou, X.H., Dong, Y., Wang, Y., Xiao, X., Xu, Y., Xu, B., Li, X., Wei, X.S. and Liu, Q.Q. (2012). A three generation study with high-lysine transgenic rice in Sprague–Dawley rats. Food and chemical toxicology, 50(6), pp.1902-1910. https://scholar.google.com/scholar?cluster=13206240425607621252
Zhou, Z.W., Wang, D. Z., Shen, X., Wang, H., Bai, J. L., Wang, X. G., & Long, W. (2012a). Comprehensive Evaluation on Functions & Safety of Imported GM Soybean Using BDI-GS System. Soybean Science. Oct. Vol. 31 No 5. Available from: http://www.ixueshu.com/document/8abbb8cc43d6fcf0318947a18e7f9386.html https://web.archive.org/web/20220116010759/https://scholar.google.com/scholar?cluster=15787145617726340247
Zhu, L., Gu, M., Meng, X., Cheung, S.C., Yu, H., Huang, J., Sun, Y., Shi, Y. and Liu, Q., (2012). High‐amylose rice improves indices of animal health in normal and diabetic rats. Plant Biotechnology Journal, 10(3), pp.353-362. https://scholar.google.com/scholar?cluster=5883870886242398264
Zhu, Y., Li, D., Wang, F., Yin, J., & Jin, H. (2004). Nutritional assessment and fate of DNA of soybean meal from roundup ready or conventional soybeans using rats. Archives of Animal Nutrition, 58(4), 295-310. https://scholar.google.com/scholar?cluster=11093005252344706248
Zhu, Y., He, X., Luo, Y., Zou, S., Zhou, X., Huang, K., & Xu, W. (2013). A 90-day feeding study of glyphosate-tolerant maize with the G2-aroA gene in Sprague-Dawley rats. Food and chemical toxicology, 51, 280-287. https://scholar.google.com/scholar?cluster=11404411338757634497
Zhuo, Q., Chen, X., Piao, J., & Gu, L. (2004). Study on food safety of genetically modified rice which expressed cowpea trypsin inhibitor by 90 day feeding test on rats. Wei sheng yan jiu= Journal of hygiene research, 33(2), 176-179. https://scholar.google.com/scholar?cluster=6833189026140238629
Zhuo, Q., Chen, X., Piao, J., & Han, C. (2004a). Study on the teratogenicity effects of genetically modified rich which expressed cowpea trypsin inhibitor on rats. Wei sheng yan jiu= Journal of hygiene research, 33(1), 74-77. https://scholar.google.com/scholar?cluster=15223846769248357182
Zinoviev, S.G. (2014). Some biochemical parameters of blood of pigs using GM soy in their diets. Animal Biology, vol. 16, № 1. https://scholar.google.com/scholar?cluster=7166204554122456608
